1“Babeş-Bolyai”
advertisement

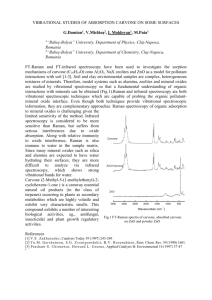
STUDIA UNIVERSITATIS BABEŞ-BOLYAI, PHYSICA, SPECIAL ISSUE, 2003 VIBRATIONAL STUDIES OF ABSORPTION CARVONE ON SOME SURFACES G.Damian1, V.Miclăuş2, I. Moldovan1, M.Puia1 “Babeş-Bolyai” University, Department of Physics, Cluj-Napoca, Romania 2 “Babeş-Bolyai” University, Department of Chemistry, Cluj-Napoca, Romania 1 ABSTRACT. FT-Raman and FT-infrared spectroscopy have been used to investigate the sorption mechanisms of carvone (C10H14O) onto Al2O3, and ZnO as a model for pollutant interactions with soil. The observed frequencies of the prominent maxima in the in the Infrared and Raman spectra of the carvone and sorbates carvone with the proposed assignments are presented. The band shifts due to adsorption effects were in the range of 15 cm-1 to low frequencies INTRODUCTION The presence of great amount of particulate matters with various composition and structures in the Earth’s atmosphere, such as insulator oxides (SiO2, Al2O3, ZnO, MgO, etc), involve the necessity of quantitative and qualitative studies on the interaction between this ones and volatile organic compounds. The chemical coupling between volatile organic compounds and particulate matter was mentioned in as of profound importance in understanding processes of the gasaerosol chemical interactions [1, 6-7]. An understanding of the mechanisms by which organic contaminants adsorb to mineral surfaces will be critical in predicting their fate and transport in the environment [8] In this paper, FT-Raman and FT-infrared spectroscopy have been used to investigate the sorption mechanisms of carvone (C10H14O) onto Al2O3, and ZnO as a model for pollutant interactions with soil [2-5]. Soil and clay environmental samples are complex, heterogeneous mixtures of minerals. Therefore, model systems such as alumina and mineral oxides are studied by vibrational spectroscopy so that a fundamental understanding of organic interactions with minerals can be obtained. Raman and Infrared spectroscopy are both vibrational spectroscopic techniques which are capable of probing the organic pollutantmineral oxide interface. Even though both techniques provide vibrational spectroscopic information, they are complementary approaches. Raman spectroscopy of organic adsorption to mineral oxides is challenging given the limited sensitivity of the method. Infrared spectroscopy is considered to be more sensitive than Raman, but suffers from serious interferences due to oxide absorption. Along with relative immunity to oxide interference, Raman is also immune to water in the sample matrix. Since many mineral oxides are expected to G.DAMIAN, V.MICLĂUŞ, I. MOLDOVAN, M.PUIA have water hydrating their surfaces, they are more difficult to analyze via infrared spectroscopy, which shows strong vibrational bands for water. EXPERIMENTAL The porous surfaces were dried four hours in air at 150oC before impregnation in order to remove free water. The samples have been prepared by stirring for two days a mixture of powder metallic oxides and liquid carvone The FT-IR spectra of carvone, surface supports and adsorbed carvone, were recorded in the region 4000-400 cm-1 by a Bruker EQUINOX 55 spectrometer, using an Attenuated Total Reflectance accessory with a scanning speed of 32 cm-1 min-1 with the spectral width 2.0 cm-1. The internal reflection element was a ZnSe ATR plate (50 x 20 x 2 mm) with an aperture angle of 45°. A total of 128 scans were accumulated for each spectrum The FT-Raman spectra were also recorded with the same instrument with a FRA 106 Raman module equipped with Nd-YAG laser source operating with 200 mW in the wave number range 3500-100 cm-1. The frequencies of all sharp bands are accurate to ±2 cm-1. RESULTS AND DISSCUSION Carvone (2-Methyl-5-(1-methylethenyl)-2-cyclohexene-1-one ) is a caraway essential natural oil products (in the class of terpenes) occurring in plants as secondary metabolites which are highly volatile and exhibit very characteristic smells. This compound exhibits a number of interesting biological activities, e.g., antifungal, insecticidal and plant growth regulatory activities. Chemical structure of the studied carvone is presented in Figure 1. Commercial metal oxides with the following specific surface (m2/g) were used as surface supports: γ-Al2O3 –200, ZnO - 6.5 The recorded FT-IR and FT-Raman Fig.1 Chemical structure of spectra of liquid carvone and adsorbed carvove carvone on Al2O3 and ZnO are shown in Figures 2 and 3. The observed frequencies of the prominent maxima in the in the infrared and Raman spectra of the carvone and sorbates studied carvone with the proposed assignments are summarized in Table 1. Assignments were made on the basis of relative intensities, magnitude of frequencies, as well as literature data of molecules of similar structure. The band shifts due to adsorption effects were in the range of 15 cm-1 to low frequencies. The C–H stretching vibrations (around 2800 cm-1 and 3100 cm-1 ) and the C–C deformation vibrations (1367 cm-1 ) are very similar for all aromatic compounds. Also, the characteristic bands of the ring vibrations (1060 cm-1) were used for identification of the sorbed products. VIBRATIONAL STUDIES OF ABSORPTION CARVONE ON SOME SURFACES Carvone ZnO+Carvone ZnO Al2O3+Carvone Al2O3 3500 3000 2500 2000 1500 1000 500 -1 Wavenumber (cm ) Fig.2 FT-Raman spectra of carvone, absorbed carvone on ZnO and A2O carvone ZnO+carvone ZnO Al2O3+carvone Al2O3 3250 2750 2250 1750 1250 -1 Wavenumber (cm ) Fig.3 FT-IR spectra of carvone, absorbed carvone on ZnO and A2O3 750 G.DAMIAN, V.MICLĂUŞ, I. MOLDOVAN, M.PUIA Table 1 Main observed bands in Raman and Infrared spectra and most probable vibrational assignment of carvone Observed wavenumber (cm-1) FT-Raman 3085 2983 FT-IR 3084 2970 2924 2923 2734 2731 1673 1644 1436 1367 1061 869 706 637 568 479 1673 1644 1634 1367 1058 960 702 Assignment C –H stretching aromatic C H stretch methyl C-H asym./sym. stretch aromatic C H stretch methyl C-H asym./sym. stretch aromatic C H stretch methyl C-H asym./sym. stretch C =C stretch C =C stretch C –C stretching , C-H bending C –C stretching ring vibrations ring breathing C – H out-of-plane bend C-H bend CCC out of plane bending CCC out of plane bending/ C = O out of plane bending REFERENCES [1] Z.Me n g, D. Dab d ub , J .H. Sei n feld , Science, 277, 116 (1997) [2] V.S. Za k har e n ko , Catalysis Today 39 (1997) 243-249 [3] Yu.M . Ger s he n zo n, S .G. Zve ni go ro d s k ii, B . V . Ro ze n s h tei n , Russ. Chem. Rev. 59 (1990) 1601. [4] P r ash a nt S. C hi n t a war, Ho ward L. Gr ee ne , Applied Catalysis B: Environmental 14 (1997) 37-47 [5] G. Da mi a n a nd V . Mi cl a us , Studia Universitatis Babes-Bolyai, Physica, XLV,1,(2000) [6] K. Gr ie sb a u m, M. H il ß , J . B o sc h , Tetrahedron 52, (1996) 14813 [7] a) K. Gr i esb a u m, V . Mic la u s, I. C. J u n g a nd R. - O. Q u i n ker t, Eur. J. Org. Chem., (1998) 627, b) K.Griesbaum, I.C.Jung, V.Miclaus, Environ. Sci. Technol., 32(1998) 647. [8] G. Da mi a n, O. Co z ar , V . M icl a u s, C s. P a iz s, V. Zna miro v sc h i , V. C hi s, L. Da vid , Colloids and Surfaces A, 137, (1998) 1