SupptInf (radical)-rev
advertisement

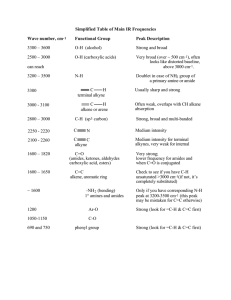
Supporting Information (SI) for 1,4-Diiodobenzene with –COO-TEMPO (TEMPO = 2,2,6,6-tetramethylpiperidine1-oxyl-4-yl) substituents at 2,5-positions: synthesis and use as a monomer for new -conjugated polymers having nitoxyl radicals in side chains By Takayuki Iijima, Masahiro Abe, Take-aki Koizumi, Atsushi Fukaya, Kyohei Usami, Kenichiro Kami, and Takakazu Yamamoto 1 (C=O) Transmittance Monomer-1 1715 (C-H) (C≡CH) 2105 (C≡C-H) Monomer-1' 3210 (-C≡C) 2204 Polymer-3 4000 3500 3000 2500 2000 1500 Wavenumber / cm 1000 -1 Figure S1a. IR spectra of Monomer-1, Monomer-1', and Polymer-3. 2 500 Transmittance 4000 3500 (C-H) (C=O) 3000 2500 2000 1500 1000 500 Wavenumber / cm-1 Figure S1b. IR spectrum of (top) Polymer-1 and (bottom) Polymer-2. The IR spectrum of Polymer-1 shows peaks due to CO2 in air at about 2350 cm-1. 3 Monomer-1 Monomer-1' Polymer-3 in CHCl3 Polymer-3 film 419 323 Absorbance 1 327 396 0 300 400 500 600 Wavelength / nm Wavelength/nm Figure S2. UV-vis spectra of Monomer-1 (green), Monomer-1' (blue), Polymer-3 in chloroform (black), and Polymer-3 in cast film (red). Annealing the Polymer-3 film at 100 ºC for 1 h under N2 showed no observable change. 4 Figure S3. ESR spectra of (solid line) Monomer-1 and (dashed line) PTMA. Figure S4. ESR spectra of Polymer-2. 5 Table S1. Crystallographic data and details of the structure refinements for Monomer-1. Monomer-1 Chemical formula C26H36I2N2O6 Formula weight 726.39 Crystal system tetragonal Space group P42/n (No. 86) a, Å 13.9179(5) c, Å V, Å 14.6048(8) 3 2829.1(3) Z μ, 4 cm-1 22.652 F(000) Dcalcd, g 1440.00 cm-3 1.705 Crystal size, mm 0.20 x 0.20 x 0.20 No. of reflns 17501 No. of reflns used 3097 No. of variables 180 R (I > 2(I)) 0.0631 R (All reflections) 0.0641 RW (All reflections) 0.1989 GOF 0.984 R1 = ||Fo|-|Fc||/|Fo| for I > 2(I) data, Rw = [ w(|Fo|-|Fc|)2/wFo2]1/2. 6