Experiment 4: Caraway and Spearmint Carvones
advertisement

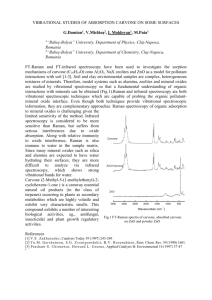
100 Chem 2315 Laboratory Manual (Experiment 4) Experiment 4: Caraway and Spearmint Carvones (Optical Activity) Pre-Lab Review Read in the Laboratory Techniques section 9. The Polarimeter and Calculation of Specific Rotation and answer the questions below. Name Lab Instructor Lab Hour Date 1. (Mark two answers.) The orange light (589.3 nm) traveling down a polarimeter whose sample cell is filled with water appears as a dull orange or dark color (as in Figure 17(c), p 36) when a. the scale reads 45.00o (polarizing filters are at 45.00o to each other). b. the scale reads 0.00o (polarizing filters are perpendicular to each other). c. the scale reads 180.00o (polarizing filters are perpendicular to each other). d. the scale reads 90.00o. (polarizing filters are parallel to each other). e. the scale reads -90.00o (polarizing filters are parallel to each other). 2. For an optically active substance whose specific rotation [á] is in the range -180 # [á] # +180o @ mL @g-1 @ dm-1, a "ghost" optical rotation reading a. is always located ±90o from the true rotation. b. always has a negative value. c. is the reading that is nearest to 0o. d. always moves towards ±180o upon dilution with an optically inactive solvent. e. always moves towards 0o upon dilution with an optically inactive solvent. 3. Which one of the statements below is false? a. Polarized light is light whose electric field wave oscillations lie in a single plane. b. An observed angle (á) to the left of zero is negative by convention. c. The observed angle (á) is independent of the optically active substance concentration in the cell. d. A solution of just one of two possible enantiomers is always “optically active.” e. The observed angle (á) for a given solution will only be half as large if the cell pathlength is cut in half. Chem 2315 Lab Manual (Experiment 4) 4. What is the correct reading on the polarimeter vernier scale shown below? a. b. c. 5. 101 43.3o 43.7o 51o d. e. 42.6o 43.15o The specific rotation [á]D23 of (+)-glyceraldehyde is +9.4o @ mL @ dm-1 @ g-1. What would be the observed rotation á of a solution of 3.00 g of (+)-glyceraldehyde dissolved in water to make a 10.00 mL solution? Assume that the sample cell is 1.00 dm long, and that the measurement was made at 23oC using the sodium D line as a light source. Show work. 102 Chem 2315 Laboratory Manual (Experiment 4) Experiment 4: Caraway and Spearmint Carvones (Optical Activity) INTRODUCTION Caraway and spearmint oils are organic extracts obtained from ground caraway seeds and spearmint leaves, respectively. Although caraway and spearmint oils each consist of about 75% carvone and 25% limonene, the two oils have quite different odors. The results of this experiment will help to explain this seemingly incongruous fact. In this experiment carvone optical activities from each oil will be examined with a polarimeter (Technique 9, p 35). The instructor will review how to operate each polarimeter. PROCEDURE Zero Correction Polarimeter 1 was zeroed precisely at the factory, so no zero correction is needed. Polarimeters 2 and 4 were zeroed at the factory, but are a little off. Student volunteers will obtain polarimeter 2 and 4 cells and clean them with distilled water and acetone. The cells are then each filled with optically inactive distilled water. Note: the screw-on ends should be tight enough that they don’t leak, but not so tight that the windows break. Be careful that the end windows do not fall out and break when emptying and filling. The volunteer students will then record zero values for polarimeters 2 and 4 and post them on the blackboard for everyone to copy into the distilled water table. This value should be subtracted from all subsequent readings on instruments 2 and 4, respectively. Polarimeter 3 was zeroed by the instructor prior to class, so no zero correction is needed. Note: please do not turn the zero knob, or it will have to be reset. Optical Rotation Since we have only four polarimeters, two volunteer students will read the pure caraway and spearmint carvone rotations (á’s). Volunteers obtain enough carvones from the stockroom to completely fill the cell. The cells should be emptied and washed with acetone before filling with carvone samples. Two readings should be made on polarimeters 1, 2 and 4 for each observed rotation angle á, one approaching clockwise and the other approaching counterclockwise the angle where both fields are dull orange or dark (as in Fig 17 (c), p 36). These two readings are Chem 2315 Lab Manual (Experiment 4) 103 then averaged. (See Techniques 9.3-6, pp 36-37.) On polarimeters 1 and 4, another reading is made ±180o from this reading. An average of this reading should be made in the same way. The positive angle á is the one of the two possible readings between 0o and +180o, while the negative reading is the one that is between 0o and -180o. Clockwise from 0o is positive, counterclockwise from 0o is negative. Observed rotations should always be between -180o and +180o. For polarimeter 2, readings can be made only in the range -90o to + 90o. For polarimeters 2 and 3, angles in the ranges -180o to -90o, or +90o to +180o are calculated by adding or subtracting 180o to observed readings between -90o and +90o.) Each undiluted carvone á value will be posted on the blackboard for all students in the class to record on the Data and Report Sheet. The two carvone densities are needed in order to calculate the specific rotation (Technique 9.7) in question 3. A student volunteer will measure each carvone density and post it for everyone to record. Methods for measuring densities are in Technique 7.2, p 29. Each student will make his/her own dilute carvone readings. Measure out about 3 g of caraway carvone into a 10 mL graduated cylinder. Record your precise mass in the blank on the Data and Report Sheet. Remember to keep the correct number of significant figures. Dilute the carvone to the 10.0 mL mark with acetone (a suitable optically inactive solvent). If you are using polarimeter 3 with the 2.00 dm cell, dilute to 20.0 mL. Use a spatula to be sure that the carvone /acetone solution is well mixed. Record your observed optical rotations (á’s) in the respective table for dilute caraway carvone. For polarimeters 1, 2 and 4, average the clockwise and counterclockwise approaches for each reading (see procedure for reading pure carvones, in the paragraph above). The clockwise/counterclockwise averaging is not necessary (or possible) on polarimeter 3 – simply record one observed á value (in the CW box). Disposal: pour used caraway carvone solution into the appropriately labeled waste bottle. The last person should completely disassemble the cell (including windows and gaskets) and wash with distilled water and acetone before returning to the stockroom. Repeat this procedure for the dilute spearmint carvone. REPORT If you used polarimeter 2 or 4, average the two zero readings (distilled water) and subtract this zero correction from all of your rotation readings (for pure and dilute carvones),. Record corrected á values in the blank provided in each table. Note: no corrections are necessary for polarimeters 1 and 3. Average the two positive corrected rotations and the two negative corrected rotations, respectively, for each undiluted carvone and record in the blank provided. Similarly, average the rotations for the diluted carvones and record for each carvone solution. Note: average á readings are not needed (calculated) for polarimeter 3. Answer the seven questions. 104 Chem 2315 Laboratory Manual (Experiment 4) EXPERIMENT 4: DATA AND REPORT SHEET Name: Lab Hour Instructor approval (initials) A volunteer will measure the zero correction for polarimeter 2. See the blackboard to fill in the following table. Distilled Water in cell Rotation (degrees) Clockwise approach Rotation (degrees) Counterclockwise approach Average of two á’s (zero correction) polarimeter #2 polarimeter #4 See the posted data sheet to fill in the following information obtained by volunteers. Density of caraway carvone. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Cell pathlength (in decimeters). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Polarimeter number used for caraway carvone. . . . . . . . . . . . . . . . . . . . . . . . . . . . . Caraway Carvone Positive Rotation (degrees) Negative Rotation (degrees) Direction of approach CW CW CCW CCW observed á corrected á average of two corrected á’s Density of spearmint carvone.. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Cell Pathlength (in decimeters). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Polarimeter number used for spearmint carvone. . . . . . . . . . . . . . . . . . . . . . . . . . . . Chem 2315 Lab Manual (Experiment 4) Spearmint Carvone 105 Positive Rotation (degrees) Direction of approach CW CCW Negative Rotation (degrees) CW CCW observed á corrected á average of two corrected á’s Mass of caraway carvone used in dilution. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Volume of caraway carvone plus optically inactive solvent. . . . . . . . . . . . . . . . . . . Cell pathlength (in decimeters). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Polarimeter number used in caraway carvone dilution. . . . . . . . . . . . . . . . . . . . . . . Caraway Carvone Dilution Positive Rotation (degrees) Direction of approach CW CCW Negative Rotation (degrees) CW CCW observed á corrected á average of two corrected á’s Mass of spearmint carvone used in dilution. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Volume of spearmint carvone plus optically inactive solvent. . . . . . . . . . . . . . . . . . Cell pathlength (in decimeters). . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Polarimeter number used in spearmint carvone dilution. . . . . . . . . . . . . . . . . . . . . . 106 Chem 2315 Laboratory Manual (Experiment 4) Spearmint Carvone Dilution Positive Rotation (degrees) Negative Rotation (degrees) Direction of approach CW CW CCW CCW observed á corrected á average of two corrected á’s QUESTIONS 1. Which one of the readings for caraway carvone (ave. value from the pure carvone table) was the true value? Explain. 2. Which one of the readings for spearmint carvone (ave. value from the pure carvone table) was the true value? Explain. 3. Using the true observed rotations for caraway and spearmint carvones (ave. values from the pure carvone tables) in eq (1) Technique 9.7, calculate the specific rotations for the two carvones. Show your calculations with unit cancellations. specific rotation (caraway carvone) specific rotation (spearmint carvone) Chem 2315 Lab Manual (Experiment 4) 4. 107 Using data from your dilution experiment and eq (1) Technique 9.7, recalculate the specific rotation of the two carvones. Note: these calculations should give the same answers as in question 3. above (within experimental error), since specific rotation is independent of the carvone concentration. Show your calculations with unit cancellations. specific rotation (caraway carvone) specific rotation (spearmint carvone) 5. Using the formula below, calculate the percent error in your specific rotations obtained from the dilution data set. Show your calculations with unit cancellations. % error in specific rotation, caraway carvone % error in specific rotation, spearmint carvone 108 6. Chem 2315 Laboratory Manual (Experiment 4) Considering the results of your optical rotation and iterature values for carvone’s specific rotation of (see below), answer the following questions. Specific rotation, carvone, = ±58.8o@mL@g-1@dm-1 Given: a. Is the carvone from caraway oil (+) or (-) rotating? (check one) (+) b. Is the carvone from spearmint oil (+) or (-) rotating? (check one) (+) 7. (-) (-) One of the carvone samples in this experiment came from caraway seed, while the other came from spearmint leaves. To about 95% of all people, the two carvones have very different odors. Explain why the carvones smell differently (to most people). (Hints: Enantiomer physical properties are identical except for the direction in which they rotate plane polarized light. Enantiomer chemical properties (with achiral reagents) are also identical.) Suggest what happens at the molecular level.