Instructor`s Copy Lab Worksheet
advertisement

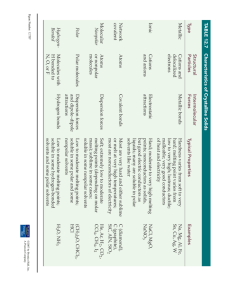
Instructor’s Copy Lab Worksheet – Polar Water Observations: Substance Sodium chloride Table 1: Compound Solubility in Water Formula Physical State NaCl Solid Solubility in Water Soluble Glycerin C3H8O3 Liquid Miscible Sucrose C12H22O11 Solid Soluble Sodium thiosulfate N2S2O3 Solid Soluble Calcium carbonate CaCO3 Solid Insoluble Hexane C6H14 Liquid Insoluble Potassium sulfate K2SO4 Solid Soluble Ethanol C2H5OH Liquid Soluble Kerosene* C9-C15 hydrocarbon Liquid Insoluble Analyses and Conclusions: 1. Polar and ionic substances generally dissolve in water; nonpolar substances do not. Explain. The slight charge of the polar water molecule is attracted to the charged particles in the polar and ionic substances. This attraction helps pull the substances apart to help them dissolve. 2. Based on the fact that calcium carbonate is an ionic compound, you may be puzzled by your experimental results for this compound. Propose an explanation for the solubility of calcium carbonate. Various answers may be given but the most common is that the calcium and carbon form a strong bond so they do not pull apart easily. 3. What basis can you use to decide whether the liquids tested are polar or nonpolar? Which of the liquid substances tested are polar? Which are nonpolar? In general, if it dissolved in the water, it is polar. For polar, students may answer ethanol, potassium sulfate, sodium chloride, and sodium thiosulfate. For nonpolar, kerosene, hexane, and calcium carbonate. Accept either one for glycerin.