LAB: Polarity
advertisement

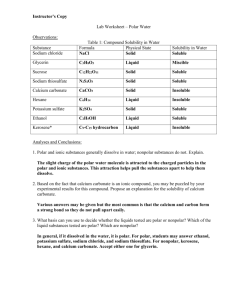
The Solvent Properties of Water Purpose: To examine the relationship between a compound’s polarity and its solubility in water. Background: “Oil and water don’t mix!” You’ve probably heard this phrase before. Though this phrase is true, there are plenty of substances that do dissolve in water. The polar nature of the water molecule is largely responsible for its remarkable solvent action. Because of its polarity, water is able to dissolve ionic compounds, such as sodium chloride and copper sulfate, and polar covalent compounds, such as sugar and ammonia. Many chemical reactions and most biochemical reactions take place in water. Similarly, many substances dissolve in oily, nonpolar solvents, such as gasoline and kerosene. In general, only nonpolar molecules will dissolve in nonpolar solvents. This is the basis for the phrase “like dissolves like.” In this experiment, you will examine the relationship between a compound’s polarity and its solubility in water. Materials: Safety goggles 10 small test tubes 1 test-tube rack 1 Wash bottle w/ DI water Sodium chloride, NaCl Sucrose, C12H22O11 Sodium thiosulfate, Na2S2O3 Calcium carbonate, CaCO3 Potassium sulfate, K2SO4 Ethanol, C2H5OH Hexane, C6H14 Glycerin, C3H8O3 Safety: 1) Wear your safety goggles 2) Ethanol and hexane are flammable. Do not use these substances near open flames. 3) Avoid skin contact with these chemicals. Many of them are toxic. 4) Follow all disposal instructions 5) Wash your hands before leaving the laboratory. Procedure: As you perform the experiment, record your results and observations in the data table. 1) Record the chemical formula, physical state, and color of each of the substances in the Materials section, with the exception of distilled water. 2) Test each of the substances for water solubility. Add 3-4 mL of distilled water to each small test tube. Add a very small quantity of the substances to be tested to an individual test tube. For solids, use a sample about the size of a match head. For liquids, use one drop. Be careful not to contaminate the chemicals with one another. Flick the test tube gently and note what happens. If all of the substance dissolves, add another small quantity and flick gently. Repeat the process several more times if the material continues to dissolve. Describe each substance as insoluble, slightly soluble, or very soluble, based on its behavior. Record these descriptions in the data table. 3) Dispose of the material as directed. Rinse the test tubes and store upside down for the next class. Name:_______________________ Lecture Teacher:__________ Period______ Pre-lab Questions: 1. Explain why water is such an excellent solvent. 2. Using the phrase “like dissolves like,” explain why gasoline and oil do not dissolve in water. 3. Define the terms solvent and solute. 4. Which substances used in this experiment are flammable? Data Table: Results and Observations Substance Formula Physical State Sodium chloride Glycerin Sucrose Sodium thiosulfate Calcium carbonate Hexane Potassium sulfate Ethanol Solubility in Water Analysis and Conclusions: 1. Polar and ionic substances generally dissolve in water; nonpolar substances do not. Explain. 2. Based on the fact that calcium carbonate is an ionic compound, you may be puzzled by your experimental results for this compound. Propose an explanation for the solubility of calcium carbonate. 3. What basis can you use to decide whether the liquids are polar or nonpolar? Which of the liquid substances tested are polar? Which are nonpolar? 4. Give the structural formulas of the liquids that were tested in this experiment. Do these formulas support the solubility data obtained? Explain. Glycerin (C3H8O3) Hexane (C6H14) Ethanol (C2H5OH 5. Based on the results of this lab, develop a hypothesis about how soaps and detergents are able to emulsify oils and greases in a solution of water. 6. Sodium chloride readily dissolves in water, however it does not dissolve in benzene. Explain why this happens.