TLW 7e Chapter 2 Answers
advertisement

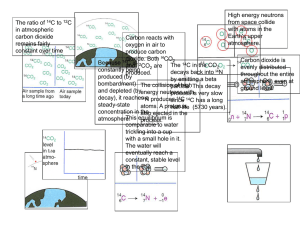
TLW 7e Chapter 2 Answers The Chemistry of Life Apply Your Understanding 1. Figure 2.8 Based on the figure, explain why it is difficult to use carbon-14 dating on 100-million-year-old dinosaurs. In order to assess a radiocarbon date you must be able to estimate the ratio of 14C to 12C in the object. After 5,730 years, it has about half the amount of 14C it had when it died. This was not a large number to begin with. After 11,460 years (the example in Figure 3.7,) there was only onefourth the amount of 14C there was when the organism died. After the organism has been dead for 50,000 years, there is approximately 1/512 the amount of 14C it started with. This is such a small amount that the measurement becomes very inaccurate. 2. This figure shows an oxygen atom forming covalent bonds with two hydrogen atoms. A carbon atom, like oxygen, has two electrons in its innermost shell, but only four electrons in its outermost shell. Using this water molecule and page 39 as guides, draw a diagram showing how carbon forms covalent bonds with two oxygen atoms in a carbon dioxide (CO2) molecule. 3. Figure 2.10 This insect is walking on water. Why doesn’t it sink? The hydrogen bonds provide a sufficiently strong surface that can support a small amount of weight; some small insects are able to walk across it without sinking. Synthesize What You Have Learned 1. Imagine you were to find a fossilized animal bone containing one-eighth the amount of carbon-14 present in Earth’s atmosphere today. How long ago did the animal die? The half-life of carbon-14 is 5,730 years. It took 5,730 years for the carbon-14 to half itself. Then another 5,730 years for that half to half itself (now ¼ left) and another 5,730 years to half the ¼ (=1/8), therefore the fossil is at least 15,190 years old. 2. Explain why it is possible for a bacterium to break the triple covalent bond of N2 gas, whereas you and other animals cannot. Bacteria have certain enzymes not present in humans that allow them to break the covalent bonds between nitrogen atoms. 3. You are on a 10-day backpacking trip with a small group of friends. Yvonne has washed out a set of water bottles with bleach. Before she can rinse them, Carlos, hot and thirsty, picks one up and drinks it, drops it, and begins to choke. What is the problem, and what can you do for him? Bleach has a pH of between 12 and 13, much too high for the delicate tissues of the mouth and throat. It can cause serious damage. In order to neutralize the bleach Carlos swallowed you could give him lemon juice or orange juice, with a pH of 2, to counteract the high pH of the bleach. You could give him milk or yogurt or even plain water to help dilute the bleach. A bicarbonate of soda or a buffer (Alka-Seltzer, Tums, or similar antacid) would also help.