06.01 Atoms, Elements, and Compounds
advertisement

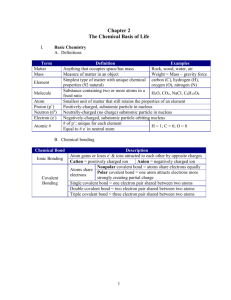
Biology Study Guide 06.01 Atoms, Elements, and Compounds SB4 Rev. 07-14-15 Online textbook: Biochemistry Name __________________________ Period __________________________ Date____________________________ Write the complete correct definition for each term. -25 for incomplete, blank, or silly answers. A. atom _____________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ B. compound ________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ C. covalent bond _____________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ D. electron __________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ E. element __________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ F. ion ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ G. ionic bond ________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ H. isotope ___________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ I. mixture ___________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ J. molecule __________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ K. neutron __________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ L. nucleus ___________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ M. proton ___________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ N. substance _________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ O. van der Waals forces ________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Also know: When a metal element and a nonmetal element combine, ionic bonds are formed. When a nonmetal and a nonmetal element combine, covalent bonds are formed. Covalent bonds may be polar (when electrons are shared unevenly) or nonpolar (when electrons are shared evenly). Polar covalent bonds make molecules magnetic. About 80% of the known elements are metals. Elements that have characteristics of both metals and nonmetals are called metalloids. Hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, iodine, and astatine usually are found in nature as diatomic molecules (two atoms combine to make one molecule). Two Group 17 elements, like fluorine atoms, will bond with each other with a single covalent bone. Two Group 16 elements, like oxygen atoms, will bond with each other with a double covalent bond. Two Group 15 elements, like nitrogen atoms, will bond with each other with a triple covalent bond. Covalent, ionic, and metallic bonds occur between atoms, inside of molecules or formula units. Van der Waals forces occur between molecules. H2O (water) is a compound formed by covalent bonds, because it is formed from two nonmetals. NaCl (salt) is a compound formed by ionic bonds, because it is formed by a metal and a nonmetal. Cu (copper) and Fe (iron) are metals; both metals are elements held together by metallic bonds.