Chapter 5
advertisement

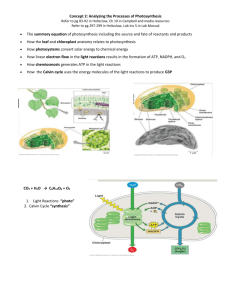
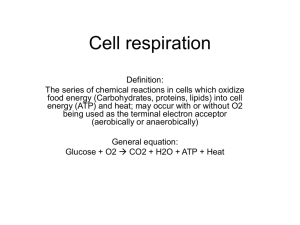
MICROBIOLOGY (BIOL& 260) CHAPTER 5 MICROBIAL METABOLISM METABOLISM Anabolic reactions 1. synthesis reactions 2. require energy 3. A + B + energy --> AB Catabolic reactions 1. breakdown reactions 2. release energy 3. AB --> A + B + energy ENZYMES Biological catalysts Reduce activation energy Enzymes are not consumed in the reaction Enzyme names end in -ase COMPONENTS OF ENZYMES Apoenzyme--protein portion produced by the cell Cofactor--inorganic “partner” (mineral) Coenzyme--organic “partner” (vitamin) Holoenzyme--whole, functioning enzyme Includes apoenzyme with the cofactor or coenzyme ENZYMATIC ACTIVITY Substrate is the substance which binds to and is changed by the enzyme The substrate binds to the active site to create the enzyme-substrate complex Products are released from the active site ENZYME ACTIVITY FACTORS 1. Temperature--optimum temp. a. too high--denaturation of enzyme b. too low--decrease in molecular kinetics 2. pH--optimum pH a. too acidic (low pH)--denaturation b. too alkaline (high pH)--denaturation 3. Substrate Conc.--increase in enzymatic activity until saturation point 4. Inhibitors Competitive inhibitors--bind to the active site; chemical structure is similar to the substrate Noncompetitive inhibitors--bind to the allosteric site; cause the active site to change its shape FEEDBACK INHIBITION Provides control over enzymatic activity End-products of an enzyme series inhibit (as allosteric inhibition) previous enzymes of the series Prevents overproduction of end-products OXIDATION AND REDUCTION Energy production often occurs via the loss (oxidation) or gain (reduction) of electrons Electron transport chain is an example RIBOZYMES Catalysts RNA molecules Substrate molecules aere RNA strands PHOSPHORYLATION 1. Substrate level phosphorylation--a phosphorylated compound transfers a phosphate to ADP to form ATP; glycolysis 2. Oxidative phosphorylation--final electron acceptor is oxygen (or another inorganic compound); electron transport chain 3. Photophosphorylation--conversion of light energy to chemical energy (ATP and NADPH); photosynthesis CARBOHYDRATE CATABOLISM 1. Glycolysis 2. Kreb’s cycle (tricarboxylic acid cycle) 3. Electron transport chain GLYCOLYSIS Also called the Embden-Meyerhof pathway Glucose enters Anaerobic Requires addition of 2ATP molecules Produces a net of 2 ATP and 2 NADH End products are 2 pyruvic acid molecules Occurs in the cytoplasm KREBS CYCLE Produces carbon dioxide Produces 2ATP molecules (one per cycle) Produces NADH Produces FADH2 Occurs in the mitochondria ELECTRON TRANSPORT CHAIN Converts NADH and FADH2 into ATP Series of oxidation and reduction reactions in proteins embedded in the inner mitochondrial membrane One NADH yields approx. 3 ATP molecules One FADH2 yields approx. 2 ATP molecules CHEMIOSMOSIS Protons are pumped out of the cell by means of the proteins of the electron transport chain A proton-motive force is created Protons are only allowed to diffuse across the membrane through certain channels (ATP synthase) which drives the production of ATP ALTERNATE METABOLIC PATHWAYS 1. Pentose Phosphate Pathway 2. Enter-Doudoroff Pathway 3. Anaerobic respiration 4. Fermentation PENTOSE PHOSPHATE PATHWAY Cyclic means of breaking down glucose to yield precursors to other compounds: amino acids glucose from carbon dioxide nucleic acids NADPH (12 from one glucose molecule) Can produce only one glucose molecule ENTER-DOUDOROFF PATHWAY Glucose to pyruvic acid Produces 2 NADPH molecules Produces 1 ATP molecule Typically not found in gram-positive bacteria ANAEROBIC RESPIRATION Use of another inorganic molecule as an electron acceptor besides oxygen: 1. nitrate ion 2. nitrite ion 3. nitrous oxide 4. nitrogen gas 5. sulfate 6. carbonate FERMENTATION Releases energy from various organic compounds Doesn’t require oxygen Doesn’t require Kreb’s cycle or electron transport chain Uses organic molecule as electron acceptor Produces modest amounts of ATP LIPID CATABOLISM Lipids are broken down by lipases Products enter glycolysis or the Kreb’s cycle PROTEIN CATABOLISM Extracellular proteases break down protein Amino acids are deaminated (this releases ammonium ions-NH4+) Some amino acids have their -COOH group removed (decarboxylation) Resultant products enter the Kreb’s cycle PHOTOSYNTHESIS Overall equation is the reverse of cellular respiration Light reactions (photophosphorylation) Light independent reactions LIGHT REACTIONS 1. Cyclic photophosphorylation 2. Noncyclic photophosphorylation CYCLIC PHOTOPHOSPHORYLATION Photon of light energizes the electron Electron is passed through electron carriers Protons are pumped across the membrane Chemiosmosis produces ATP from ADP Excited electron ultimately returns to where it began (chlorophyll) NONCYCLIC PHOTOPHOSPHORYLATION Photon-excited electrons pass through electron carriers to produce ATP from proces of chemiosmosis Electrons become incorporated into NADPH Electrons from water replace these electrons Oxygen is released CALVIN-BENSON CYCLE (LIGHT INDEPENDENT) Similar to the Kreb’s cycle except carbon dioxide goes in and glyceraldehyde 3phosphate is produced (two such molecules can be converted into glucose) To make one glucose the cycle must turn 6 times Requires 6 carbon dioxide, 18 ATP, and 12 NADPH NUTRITIONAL TYPES Photoautotrophs (plants)--obtain energy from the sun, obtain carbon from carbon dioxide Photoheterotrophs (some species of nonsulfur bacteria)--obtain energy from light, obtain carbon from organic compounds Chemoautotrophs (hydrogen, sulfur, iron and nitrifying bacteria)--obtain energy from inorganic compounds, obtain carbon from carbon dioxide Chemoheterotrophs (ourselves)--obtain energy from organic compounds, carbon from organic compounds LEARNING OBJECTIVES FOR CHAPTER 5 Following successful study of this chapter, students should be able to: 1. Define or describe each of the following terms: metabolism, catabolism, anabolism, metabolic pathways, enzymes, apoenzyme, cofactor, coenzyme, holoenzyme, activation energy, enzyme-substrate complex, substrate, active site, allosteric inhibition, feedback inhibition, ribozymes, oxidation, reduction, redox reactions, aerobe, anaerobe, photoautotrophs, photoheterotrophs, chemoautotrophs, chemoheterotrophs 2. List and describe the factors which affect enzymatic activity (eg. temperature, pH, substrate concentration, competitive inhibitors, noncompetitive inhibitors) 3. List and describe the various types of phosphorylation: substrate-level phosphorylation, oxidative phosphorylation, photophosphorylation. 4. Describe the general characteristics for each of the following metabolic pathways: glycolysis, pentose phosphate pathway, Entner-Doudoroff pathway, Krebs cycle, electron transport chain, chemiosmosis, anaerobic respiration, fermentation, cyclic photophosphorylation, noncyclic photophosphorylation, Calvin-Benson cycle,