Microbial Metabolism Catabolic and Anabolic Reactions
advertisement
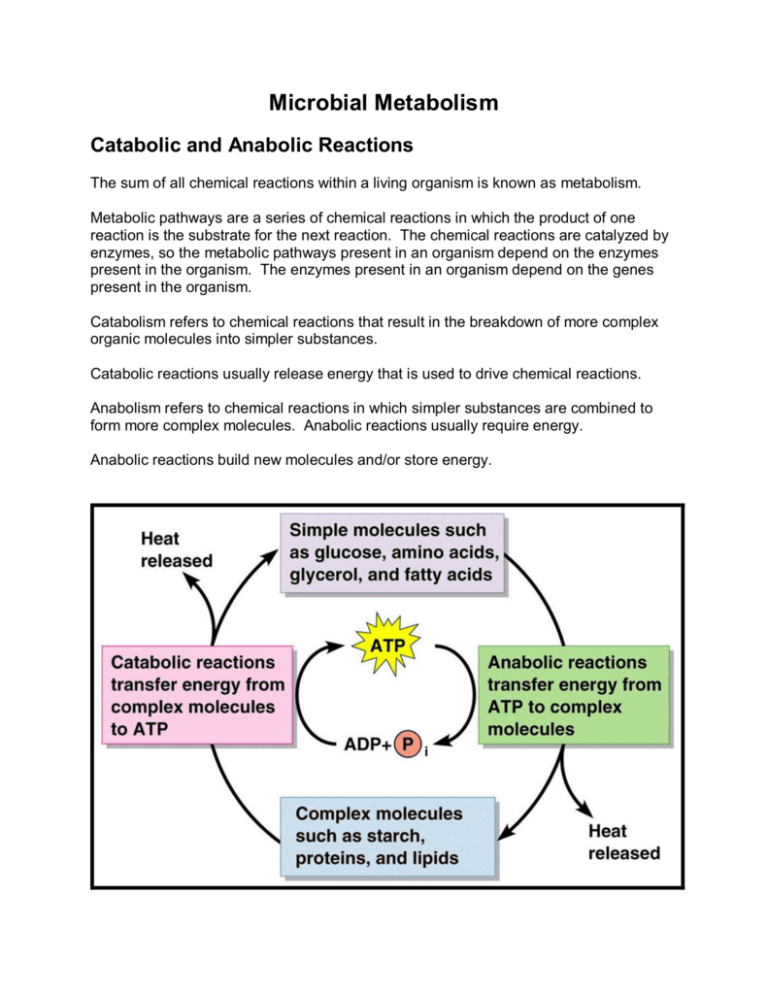
Microbial Metabolism Catabolic and Anabolic Reactions The sum of all chemical reactions within a living organism is known as metabolism. Metabolic pathways are a series of chemical reactions in which the product of one reaction is the substrate for the next reaction. The chemical reactions are catalyzed by enzymes, so the metabolic pathways present in an organism depend on the enzymes present in the organism. The enzymes present in an organism depend on the genes present in the organism. Catabolism refers to chemical reactions that result in the breakdown of more complex organic molecules into simpler substances. Catabolic reactions usually release energy that is used to drive chemical reactions. Anabolism refers to chemical reactions in which simpler substances are combined to form more complex molecules. Anabolic reactions usually require energy. Anabolic reactions build new molecules and/or store energy. The energy of catabolic reactions is used to drive anabolic reactions. The energy for chemical reactions is stored in ATP. Often when I ask students "What is ATP" the answer they give is "Energy”. This may be a shorthand answer but what I am looking for is "Adenosine triphosphate, a molecule that contains three phosphates held together by high energy bonds. When the third phosphate is cleaved, leaving adenosine diphosphate (ADP), energy is released to drive anabolic reactions. Energy is required to add a third phosphate to ADP to form ATP; the energy comes from catabolic reactions and is stored in the newly formed bond." Or, my shorthand: "The rechargeable battery of the cell". Enzymes Enzymes are proteins, produced by living cells; they catalyze chemical reactions by lowering the activation energy. Activation energy is the energy required for a chemical reaction to occur. Enzymes are generally globular proteins with characteristic three­ dimensional shapes. The active site is the binding site for the enzyme’s substrate. Enzymes are specific for substrate molecules. This is all shape driven, so anything that alters the shape of the active site will affect enzyme activity. Enzymes are efficient, can operate at relatively low temperatures, and are subject to various cellular controls. Naming Enzymes Enzyme names usually end in –ase. The six classes of enzymes are defined on the basis of the types of reactions they catalyze. · Oxidoreductase – catalyzes redox reactions · Transferase – catalyzes transfer of functional groups · Hydrolase – splits chemical bonds by addition of water · Lyase – splits chemical bonds without using water (not a hydrolysis reaction) · Isomerase – rearranges atoms within a molecule · Ligase – forms a chemical bond between two atoms Enzyme Components Most enzymes are holoenzymes, consisting of a protein portion (apoenzymes) and a nonprotein portion (cofactor). The cofactor can be a metal ion (iron, copper, magnesium, manganese, zinc, calcium, or cobalt) or a complex organic molecule known as a coenzyme (NAD + , NADP + , FMN, FAD, or coenzyme A). The Mechanism of Enzymatic Action When an enzyme and substrate combine, the substrate is transformed into the product, and the enzyme is recovered. Enzymes are characterized by specificity, which is a function of their active sites. Factors Influencing Enzymatic Activity At high temperatures, enzymes undergo denaturation and lose their catalytic properties; at low temperatures, the reaction rate decreases. The pH at which enzymatic activity is maximal is known as the optimum pH. Within limits, enzymatic activity increases as substrate concentration increases. Competitive inhibitors compete with the normal substrate for the active site of the enzyme. Noncompetitive inhibitors act on other parts of the apoenzyme or on the cofactor and decrease the enzyme’s ability to combine with the normal substrate. Feedback Inhibition Feedback inhibition occurs when the end product of a metabolic pathway inhibits an enzyme’s activity near the start of the pathway. Ribozymes Ribozymes are enzymatic RNA molecules that cut and splice RNA in eukaryotic cells. Energy Production Oxidation­Reduction Reactions Oxidation is the removal of one or more electrons from a substrate. Protons (H + ) are often removed with the electrons. Reduction of a substrate refers to its gain of one or more electrons. Each time a substance is oxidized, another is simultaneously reduced. NAD + is the oxidized form; NADH is the reduced form. Glucose is a reduced molecule; energy is released during a cell’s oxidation of glucose. The Generation of ATP Energy released during certain metabolic reactions can be trapped to form ATP from ADP and phosphate. Addition of a phosphate to a molecule is called phosphorylation. During substrate­level phosphorylation, a high­energy phosphate from an intermediate in catabolism is added to ADP. During oxidative phosphorylation, energy is released as electrons are passed to a series of electron acceptors (an electron transport chain) and finally to O2 or another inorganic compound. During photophosphorylation, energy from light is trapped by chlorophyll, and electrons are passed through a series of electron acceptors (an electron transport chain). The electron transfer releases energy used for the synthesis of ATP. Chemiosmosis The energy from electron transfer through an electron transport chain drives proton pumps. The proton pumps move protons to one side of the membrane, increasing the concentration on one side and decreasing the concentration on the other side. The protons diffuse down their concentration gradient through a membrane channel that has enzymatic (ATPase) activity. The energy of the protons moving through the channel drives phosphorylation of ADP to make ATP. Or, here is another way to say the same thing: Generation of ATP Organisms must regenerate ATP to continue their metabolic activities. There are three basic mechanisms an organism may use to generate ATP. Mechanisms of ATP Generation Substrate­level phosphorylation – an enzyme transfers a phosphate group from a donor molecule (the substrate) to ADP to form ATP. Oxidative phosphorylation – electrons are transferred from organic compounds down an electron transport chain to a final electron acceptor; energy released during electron transfer is harnessed to make ATP by chemiosmosis. Photophosphorylation – electrons are raised to a higher energy level by light, passed down an electron transport chain to a final electron acceptor, and energy released during electron transfer is harnessed to make ATP by chemiosmosis. Two more things, both are a part of oxidative phosphorylation and photophosphorylation (call them mini­mechanisms if you want): Electron transport chain ­ a series of molecules in a membrane that pick up electrons from carrier molecules like NADH and FADH2, pass the electrons to sequentially to one another in a series of oxidation­reduction reactions, and use the energy released from the electrons to pump protons across the membrane in which they are embeded. Chemiosmosis ­ the proton­motive force generated by the electron transport chain is harnessed by ATP synthase and used to make ATP from ADP and phosphate. The processes that use the mechanisms are: Cellular respiration – ATP is generated by oxidation of organic molecules, the passage of electrons down an electron transport chain, and chemiosmosis. The final electron acceptor is almost always inorganic. Aerobic – the final electron acceptor is O2. Anaerobic – the final electron acceptor is some inorganic molecule other than O2. Fermentation – ATP is generated by oxidation of organic molecules and the final electron acceptor is an organic molecule. The electrons produced by oxidation are not used to make ATP. ATP production is accomplished by substrate­level phosphorylation. Photosynthesis – ATP is generated by photophosphorylation. Cyclic reactions produce ATP. The electrons come from chlorophyll, pass down an electron transport chain, and return to chlorophyll. Non­cyclic reactions produce ATP and NADPH. The electrons come from chlorophyll, travel down an electron transport chain, and are passed to NADP to form NADPH. Chlorophyll is then reduced by H2O or some other oxidizable compound like H2S to replace the lost electrons. Here is an overview: And here is the overview with a little more information (click here) Metabolic Pathways of Energy Production A series of enzymatically catalyzed chemical reactions called metabolic pathways store energy in and release energy from organic compounds. Carbohydrate Catabolism Most of a cell’s energy is produced from the oxidation of carbohydrates. Glucose is the most commonly used carbohydrate. The two major types of glucose catabolism are respiration, in which glucose is completely broken down, and fermentation, in which it is partially broken down. Glycolysis (Embden­Meyerhof pathway) The most common pathway for the oxidation of glucose is glycolysis. Pyruvic acid is the end­product. Two ATP and two NADH molecules are produced from one glucose molecule. Alternatives to Glycolysis The pentose phosphate pathway (hexose monophosphate shunt) is used to metabolize five­ carbon sugars; one ATP and 2 NADPH molecules are produced from oxidation of one glucose molecule. The pentose phosphate pathway produces intermediates for nucleic acid synthesis, glucose synthesis from CO2 in photosynthesis, and some amino acids. The Entner­Doudoroff pathway yields one ATP and two NADPH molecules from one glucose molecule. Entner­Doudoroff is used by Gram negatives (e.g. Rhizobium, Psuedomonas, Agrobacterium), usually not by Gram positives. Cellular Respiration During respiration, organic molecules are oxidized. Energy is generated from the electron transport chain and the final electron acceptor is an inorganic molecule. In aerobic respiration, O2 functions as the final electron acceptor. In anaerobic respiration, the final electron acceptor is an inorganic molecule other than O2. Aerobic Respiration Oxidative Decarboxylation of Pyruvic Acid (Transition Reaction) Decarboxylation of each pyruvic acid produces one CO2 molecule and one acetyl group linked to CoA (acetyl­CoA). 1 NAD is reduced to 1 NADH (2 electrons removed from pyruvate) for each pyruvate. The Krebs Cycle Two acetyl­CoA groups are oxidized in the Krebs cycle for each glucose molecule (one six carbon glucose is oxidized to two 3 carbon pyruvic acid molecules, each of which is decarboxylated to produce an acetyl­CoA molecule). Electrons are picked up by NAD + and FAD for the electron transport chain. From one molecule of glucose, oxidation in the Krebs cycle produces six molecules of NADH, two molecules of FADH2, and two molecules of ATP. Decarboxylation produces four additional molecules of CO2. The Electron Transport Chain (System) Electrons are brought to the electron transport chain by NADH. The electron transport chain consists of carriers, including flavoproteins, cytochromes, and ubiquinones. Electrons are passed from one carrier to the next, the energy is used to drive proton pumps. The final electron acceptor is irreversibly reduced; it may be oxygen (aerobic) or another inorganic molecule (anaerobic). The Chemiosmotic Mechanism of ATP Generation Protons being pumped across the membrane generate proton motive force as electrons move through a series of acceptors or carriers. Energy produced from movement of the protons back across the membrane is used by ATP synthase to make ATP form ADP and phosphate. In eukaryotes, electron carriers are located in the inner mitochondrial membrane; in prokaryotes, electron carriers are in the plasma membrane. A Summary of Aerobic Respiration In aerobic prokaryotes, 38 ATP molecules can be produced form complete oxidation of a glucose molecule in glycolysis, the Krebs cycle, and the electron transport chain. In eukaryotes, 36 ATP molecules are produced from complete oxidation of a glucose molecule (2 ATP are required to shuttle the 2 electrons from the NADH produced in glycolysis across the mitochondrial membrane to the electron transport chain). Anaerobic Respiration The final electron acceptors in anaerobic respiration include NO3 ­ , SO4 2­ , and CO3 2­ . NO3 ­ (nitrate) reduced to NO2 ­ (nitrite) SO4 2­ (sulfate) reduced to H2S (hydrogen sulfide) CO3 2­ (carbonate) reduced to CH4 (methane) The total ATP yield is less than in aerobic respiration because only part of the Krebs cycle operates under anaerobic conditions. Fermentation Fermentation is any process that releases energy from sugars or other organic molecules by oxidation, does not require O2, the Krebs cycle, or an electron transport chain, and uses an organic molecule as the final electron acceptor. Fermentation can sometimes occur in the presence of O2. Two ATP molecules are produced by substrate­level phosphorylation. Electrons removed from the substrate reduce NAD + to NADH. In lactic acid fermentation, pyruvic acid is reduced by NADH to lactic acid (lactic acid fermenters include Streptococcus and Lactobacillus). Lactic acid can be fermented to propionic acid and CO2 by Propionibacterium freudenreichii (Swiss cheese). In alcohol fermentation, acetaldehyde is reduced by NADH to produce ethanol (alcohol fermenters include yeasts and bacteria). Ethanol can be fermented to acetic acid (vinegar) by Acetobacter. Acetic acid can be fermented to methane by Methanosarcina. Heterolactic fermenters can use the pentose phosphate pathway to produce lactic acid and ethanol. Homolactic fermenters produce only lactic acid. Lipid and Protein Catabolism Lipid Catabolism Lipases hydrolyze lipids into glycerol and fatty acids. Fatty acids and other hydrocarbons are catabolized by beta oxidation. Beta oxidation produces two carbon units that are linked to CoA to make acetyl­CoA. Catabolic products can be further broken down in glycolysis and the Krebs cycle. Protein Catabolism Before amino acids can be catabolized, they must be converted to various substances that enter the Krebs cycle or glycolysis. Transamination (transfer of NH2), decarboxylation (removal of COOH), and dehydrogenation (H2) reactions convert the amino acids to be catabolized into substances that enter the glycolytic pathway or Krebs cycle. Biochemical Tests and Bacterial Identification Bacteria and yeast can be identified by detecting action of their enzymes. Acid production on the left from fermentation of glucose, alkaline end products from protein degradation on the right Fermentation tests are used to determine whether an organism can ferment a carbohydrate to produce acid and gas Photosynthesis Photosynthesis is the conversion of light energy from the sun into chemical energy; the chemical energy is used for carbon fixation. The Light­Dependent Reactions: Photophosphorylation Chlorophyll a is used by green plants, algae, and cyanobacteria; it is found in thylakoid membranes. Other bacteria us bacteriochlorophylls. Electrons from chlorophyll pass through an electron transport chain, from which ATP is produced by chemiosmosis. In cyclic photophosphorylation, the electrons return to the chlorophyll. In noncyclic photophosphorylation, the electrons are used to reduce NADP + , and electrons are returned to chlorophyll from H2O or H2S. When H2O is oxidized by green plants, algae, and cyanobacteria, O2 is produced (photosystem II, noncyclic photophosphorylation) The Light­Independent Reactions: The Calvin­Benson Cycle CO2 is used to synthesize sugars in the Calvin­Benson cycle. CO2 is ligated to ribulose diphosphate (5 carbon molecule) to produce glucose. · Three CO2 molecules and three RuDP molecules yield six glyceraldehyde­3­ phosphate molecules. · Five glyceraldehyde molecules are converted to three RuDP molecules and one glyceraldehyde 3­phosphate is ligated to another to form glucose. To produce 1 molecule of glucose 6 molecules of CO2, 18 molecules of ATP, 12 molecules of NADPH, and 6 molecules of H2O are required. A Summary of Energy Production Mechanisms Sunlight is converted to chemical energy in oxidation­reduction reactions carried on by phototrophs. Chemotrophs can use this chemical energy. In oxidation­reduction reactions, energy is derived from the transfer of electrons. To produce energy, a cell needs an electron donor (organic or inorganic), a system of electron carriers, and a final electron acceptor (organic or inorganic). Metabolic Diversity Among Organisms Carbon Source: Autotrophs are self­feeders and use CO2 as a carbon source. Heterotrophs feed on others and use organic sources of carbon. Energy source: Phototrophs use light as an energy source. Chemotrophs use redox reactions of organic or inorganic compounds as an energy source. Photoautotrophs obtain energy by photophosphorylation and fix carbon from CO2 via the Calvin­Benson cycle to synthesize organic compounds. Cyanobacteria are oxygenic phototrophs (noncyclic). Green sulfur bacteria and purple sulfur bacteria are anoxygenic phototrophs (cyclic). Photoheterotrophs use light as an energy source and an organic compound for their carbon source or electron donor – can’t fix CO2. Chemoautotrophs use inorganic compounds as their energy source and carbon dioxide as their carbon source. Chemoheterotrophs use complex organic molecules as their carbon and energy sources. Metabolic Pathways of Energy Use Polysaccharide Biosynthesis Glycogen is formed from ADPG (ATP + glucose 6­phosphate = adenosine diphosphoglucose) in bacteria and from UDPG in animals (UTP + glucose 6­phosphate = uridine diphosphoglucose). UDPNAc is the starting material for the biosynthesis of peptidoglycan (UTP + fructose 6­ phosphate = UDP­N­acetylglucosamine). Lipid Biosynthesis Lipids are synthesized form fatty acid and glycerol. Glycerol is derived from dihydroxyacetone phosphate, and fatty acids are built from acetyl CoA. Amino Acid and Protein Biosynthesis Amino acids are required for protein biosynthesis. All amino acids can be synthesized either directly or indirectly from intermediates of carbohydrate metabolism, particularly from the Krebs cycle. Transamination or amination reactions: Organic acids + an amine group = amino acid. Not all organisms can do this. Some require preformed amino acids. Purine and Pyrimidine Biosynthesis The sugars composing nucleotides are derived from either the pentose phosphate pathway or the Entner­Doudoroff pathway. Carbon and nitrogen atoms from certain amino acids (aspartic acid, glycine, glutamic acid) form the backbones of the purines and pyrimidines. Includes DNA, RNA, ATP, NAD, NADP, FMN, and FAD. The Integration of Metabolism Anabolic and catabolic reactions are integrated through a group of common intermediates. Such integrated metabolic pathways are referred to as amphibolic pathways.