BACTERIA LAB – Sampling, Isolation, and Antibiotic Sensitivity
advertisement

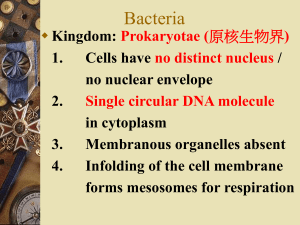
BACTERIA LAB – Sampling, Isolation, and Antibiotic Sensitivity Testing of Gram +/_ bacteria. INTRODUCTION: The bacteria we will work with are hiding in plain sight - on our skin, on our food, living in your kitchen sponge and in your toothbrush. They are thriving in sink drains and on the surface of all living things, but they are most abundant in soil, where billions of organisms can be found in one cubic centimeter. These "intimate strangers" will become a bit more familiar to you this week. Common bacteria are traditionally separated into two main groups, those with gram positive cell walls (G+) and those with gram negative cell walls (G-). Clever scientists have devised simple means to "fish" these out separtely by developing growth media that are selective for the growth of one type or the other. The media is made from a mixture of specific nutrients and made semi-solid by adding a gelling agent called agar. Agar is extracted from red algae. It is nutritionally inert. Agar is fascinating on its own. At 1.5%, it causes water-based solutions to gel. It melts/dissolves at 100 degrees, and remains liquid until its temperature drops below 40 degrees. Conversely, when the 1.5% agar gel is heated, it remains solid until its temperature is increased to over 100 degrees. Therefore, the 1.5% agar-based media can be liquid or solid between 40 and 100 degrees depending on whether it is being heated or cooled. This is extraordinary all by itself. *Monday: You will have petri dishes of agar-based media. Monday's activity will employ two different formulas on a petri plate that is divided down the center. On one half of the biplate is Brilliant Green Agar (BGA), which supports the growth of only gram negative bacteria to the exclusion of gram positive bacteria. The other half of the plate contains Phenylethylalcohol Agar (PEA), which is selective for only gram positive bacteria to the exclusion of gram negative bacteria. The media has been sterilized in the autoclave, so initially there is nothing alive within the plates. To recover bacteria, we need only use a sterile swab, rub the area to be sampled, then gently rub the swab over the surface of the media. Incubation at 37 degrees for 24 hours will offer the bacteria the opportunity to reproduce exponentially with abundant nutrients and little or no competition. You will not be able to see the thousands of cells swabbed onto the plates. *Tuesday: You will see hundreds of billions of cells growing on the plates. Those recovered on BGA will be gram negative and those recovered on PEA will be gram positive. You should make careful observations of the resulting growth considering in each case the source of your sample. Look for any trends by comparing your results with those of your colleagues. Typically, you will recover numerous species, and for further study, these species should be separated (isolated) by a technique called the streak plate. It should be noted that the majority of bacteria are not cooperative, and will not grow on the media we use. It's like fishing with different bait to catch different kinds of fish, but knowing full well that there were fish that refused either type of bait. Your collegeeducated teacher will demonstrate the streak plate method using media that is nonselective (supports both gram positive and gram negative organisms) - in this case, Tryptic Soy Agar (TSA). The streak plates will be incubated for 24 hours at 37 degrees. *Wednesday: We will have several different gram positive bacteria and several different gram negative bacteria isolated and ready for a controlled experiment. We will perform the Kirby-Bauer Antibiotic Sensitivity Test. Each lab group will compare the reactions of one G+ and one G- organism to Penecillin, Kanamycin, and Vancomycin. Each is a common antibiotic derived from fungi. Since fungi and bacteria are the decomposers of the world, they compete for food. The competition is fierce, and each employs chemical warfare to keep its competition at bay. The KBAST involves selecting an isolated colony of cells with a sterile swab, and dropping it into a tube with 10 ml of sterile water. The cap is then returned to the tube and the tube is vigorously shaken using a vortex mixer. The cells become uniformly suspended in the water. Then the cap is removed, and the swab is used to swab the surface of a plate of Mueller Hinton Agar (MHA). MHA is THE media used for antibiotic sensitivity testing. After the surface of the MHA plate is thoroughly swabbed with the suspension of cells, a paper disc impregnated with the antibiotics listed above is carefully placed on the surface. The antibiotic diffuses out of the disc and into the MHA. These plates are incubated for 24 hours. So you will have 2 plates per group, one with a G+ bacterium, and one with a G- bacterium, and each with 3 antibiotic discs. *Thursday: The plates will be examined, and you will measure the zones of inhibited growth around each of the discs. The diameter of the zone of inhibition is used to interpret whether the bacterium is sensitive or resistant to the antibiotic in question. Again, comparing your group's results with those of your colleagues will allow you to make some conclusions, albeit limited, regarding the differential reactions of G+ and Gorganisms to these antibiotics. You may then perhaps infer something about the G+ and Gcell walls of bacteria with regards to any protection they may confer against these antibiotics.