Advanced Chemistry Notes - Bridgman Public Schools
advertisement

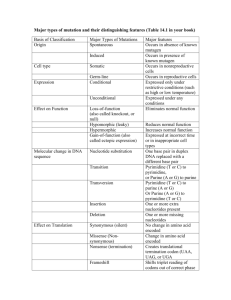
Advanced Chemistry Notes Biochemistry Vocabulary: Biochemistry the study of chemistry in living (biological) systems Macromolecule big molecule Polymer a very large molecule containing many atoms arranged in repeating units Monomer single repeating init of a polymer Recall from Organic Chemistry We began by saying organic compounds were made from aliphatic and aromatic hydrocarbons We then added functional groups to these carbons compounds We will now look at organic compounds when they are joined at the functional groups to make macromolecules Organic reactions: this is how organic compounds are joined together Condensation reactions macromolecules are formed by the removal of water o Ex: Glucose and fructose produce sucrose in this type of reaction o Ex: Propanoic acic and ethanol Hydrolysis reactions macromolecules are broken down by water (opposite of condensation reactions o Ex: the hydrolysis of diethyl amine Classifications of macromolecules: Carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates: general formula (CH2O)n, consist of mono-, di-, and polysacchrides o Monosaccharides – (simple sugars) 3 – 7 carbons Can be bonded together to make polysaccharides Function – to store energy Energy is stored in bonds and released when bonds break Ex: Glucose, fructose, glalactose. o Disaccharides – two monosaccharides bonded together Ex: Sucrose (see above diagram) o Polysaccharides – many monosaccharides bonded together Example of polysaccharides: Glycogen – animal energy storage Starch – plant energy storage Cellulose – plant cell walls (fiber) Chitin – cell wall of fungi and exoskeletons of arthropods Lipids: consist of a glycerol and fatty acids o Fatty acids – a long hydrocarbon chain with a carboxyl (acid) group. The chain is usually 16 to 18 carbons long Saturateded – have all single bonded carbons Unsaturated – have at least one double bond carbon Unsaturated fatty acids have bends to them and cannot be packed as close together therefore these molecules are less dense These less dense fatty acids have lower melting points o Ex: butter contains more saturated fat then corn oil, and is a slid at room temperature while corn oil is a liquid o Phospholipids – have structures like triglycerides but contain a phosphate group instead of a third fatty acid Phospholipids have a polar head (PO42-) and a nonpolar tail (fatty acid) This causes phospholipids to spontaneously form layers in a watery environment Cell membranes are made up of phospholipid bilayers The Fluid Mosaic Model – Proposed in 1972 The model describes the lipid bilayer of vesicles as a dynamic, liquid-like environment. The model also characterizes the lipid bilayer as a complex mixture of both phospholipids and proteins. Both the phospholipids and the proteins may be further conjugated with other groups, such as carbohydrates. Membrane Proteins - Proteins associated with the lipid bilayer are of two general types: Peripheral or Extrinsic membrane proteins. These proteins are polar proteins and associate with the polar head groups of the bilayer surface via hydrogen bonds and ionic interactions. They do not penetrate the hydrophobic region of the bilayer, and can be dissociated from the surface under buffer conditions that disrupt ionic interactions (e.g. pH, and high salt) Intrinsic membrane proteins. Contain hydrophobic regions or surfaces that penetrate within the hydrophobic region of the lipid bilayer. Integral membrane proteins may extend partly into the hydophobic region, or may span the entire bilayer and have polar regions that face the aqueous environment on both the inside and outside surfaces. Because of their penetration into the hydrophobic region of the bilayer, integral proteins are not readily separated from the bilayer, and require the addition of detergents to remove and solubilize them. The lipid bilayer is about 5nm thick (50Å). Thus, an intrinsic membrane protein that spans the membrane has a length on the order of 5nm Proteins – consists of amino acid monomers o Functions of proteins: enzymes (chemical reactions); hormones; storage (egg whites of birds, reptiles; seeds); transport (hemoglobin); contractile (muscle); protective (antibodies); membrane proteins (receptors, membrane transport, antigens); structural; toxins (botulism, diphtheria) o Amino Acids (building blocks of protein) – organic molecules that consist of a carboxyl (acid) group and a amine group Each amino acid is different and therefore has it own properties Amino acids are joined together by a peptide bond that results from a condensation reaction. Peptide – two or more amino acids joined Polypeptide – chain of amino acids Protein – a complete product of one or more polypeptides Overview of our progression to proteins is below: Atoms (C) Organic Compounds (CH4) Organic Compounds w/ Functional Groups (R-COOH) Organic Compounds with an amine and carboxyl group (amino acids) Amino acid connected (peptides) Chains of amino acids (polypeptides) Chains of amino acids connected (proteins). The following is the list of amino acids in one of the polypeptide chains of hemoglobin. (Protein that carries oxygen in your blood) val his leu thr pro glu glu lys ser ala val thr ala leu tyr gly lys val asn val asp glu val gly gly glu ala leu gly arg leu leu val val tyr pro try thr gln arg phe phe glu ser phe gly asp leu ser thr pro asp ala val met gly asn pro lys val lys ala his gly lys lys val leu gly ala phe ser asp gly leu ala his leu asp asp leu lys gly thr phe ala thr leu ser gln leu his cys asp lys leu his val asp pro glu asn phe arg leu leu gly asn val leu val cys val leu ala his his phe gly lys glu phe thr pro pro val gln ala ala tyr gln lys val val ala gly val ala asp ala leu ala his lys tyr his The following is the amino acid alanine (ala). This is one of the amino acids in hemoglobin. Hemoglobin consists of four polypeptide chains o o o o Protein structure: The large number of charged atoms in a polypeptide chain facilitates hydrogen bonding within the molecule, causing it to fold into a specific 3-dimensional shape. The 3-dimensional shape is important in the activity of a protein. Enzymes: proteins that speed up chemical reactions (catalysts) Ex: Pepsin – breaks down proteins Lipase – breaks down lipids Amylase – breaks down carbohydrates Other kinds of proteins: Some proteins contain only amino acids Gycoproteins contain carbohydrates Lipoproteins contain lipids Denaturing: occurs when the normal bonding patterns are disturbed causing the shape of the protein to change Proteins can be natured by changes in the following Temperature Acidity Salt concentration