Material Properties
advertisement

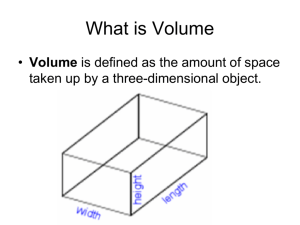
Dublin High School Honors Chemistry Alchemy Pennies - “All That Glitters” Measuring with Significant Figures PURPOSE: It is not always easy to tell one form of matter from another. This lab activity will show you how to differentiate between forms of matter by examining density, one of the physical properties of matter. Density = Mass / Volume 1. Remember that you are responsible for the safety of yourself and your lab partners. You may be prohibited from any further laboratory activities if you do not follow common sense safety precautions or if you are seen playing with any measuring device or other materials. 2. Any or all of the information in this lab may be seen again on a quiz, test, or some other format. Know and understand each question. Takes notes if appropriate and make a “foldie” for each word in bold. A. MEASURING LENGTH 1. Examine a meter stick (ruler). Notice that it is divided into one hundred equal-numbered parts. Each numbered division is one centimeter (cm) long. Thus, one centimeter is 0.01 meters. Each centimeter is divided into ten equal non-numbered parts called millimeters (mm). Therefore, one millimeter is 0.001 meters. The length along a meter stick between 10.0 cm and 12.5 cm would be 2.5 centimeters. This same length could also be expressed as 25 millimeters, 0.025 meters, or 2.5 x 10-2 meters. When you make measurements with a ruler or meter stick, you should never use the ends because they may be damaged or stamped improperly. For a more accurate reading, use another marking farther along the ruler. (See the figure to the right.) Be sure to subtract your reading of the first mark from the last mark, as in the figure. When measuring with a meter stick or ruler, notice that it is placed on/along its metric side. This helps to eliminate errors that can arise by viewing the scale from different angles, called parallax. Finally, when making any scientific measurements, notice that we always estimate a last digit in between the smallest increments marked on the device. Thus in the figure above, the first mark is at 7.35 centimeters, not simply 7.3 or 7.4 centimeters. The 0.05 was estimated to a tenth in between the 7.3 and 7.4 centimeters. (Note: The final estimated digit and all figures to the left of it are significant figures.) B. MEASURING MASS AND VOLUME 1. Mass refers to the quantity of matter composing an object. To find the mass of an object, we can compare its mass with a known mass. This is what any type of “balance” does. All balances must first be zeroed by adjusting a movable nut. To find the mass of an object, we could also measure the amount that a wire, spring, or other object is moved by a mass. Through a known scale of conversion, digital scales calculate and display the mass that would have such an effect. Inspect your digital scale. These scales are VERY expensive ($200-$300) and VERY fragile. You must always exercise extreme care when working with them, as you will be held responsible for any damage. Too much jostling around will cause them to become irreparably inaccurate. Plug in the scale carefully and turn it on. Wait a second or two for it to power up. You must always check that the scale is reading in grams when it first comes up. Do so now. Just as with balances, scales also need to be zeroed. This is sometimes called the “tare”. Press “tare” or “eraser” (sometimes combined with the ON button) to make sure your electronic scale is zeroed out. (Note: Even though these cost a couple of hundred dollars, they are still "cheap" as electronic scales go. That means that sometimes they don't settle very well. If this happens, everyone should make sure that they are in no way touching the table and that there is no breeze when you are trying to read the mass. 2. Using the balance, determine the mass of the 40 pennies minted prior to 1982. Repeat this measurement two more times and record in Table A. Average the results of the three trials to determine the average mass of the pennies. 3. Repeat step two with pennies minted after 1982 and record those results in Table A. 4. Volume is always measured with a graduated cylinder, never a beaker or any other device. This is because no other device is as accurate as a graduated cylinder. Also, the smaller the diameter of the graduated cylinder, the more accurate the measurement. This is one reason beakers are not very accurate, as they have much larger diameters. Graduated cylinders should always be carefully laid on their sides when not in use, because if they are standing up and get knocked Dublin High School Honors Chemistry Alchemy Pennies - “All That Glitters” Measuring with Significant Figures over they will certainly break! The volume of a liquid is determined by pouring that liquid directly into the graduated cylinder. The volume of a solid is determined by using the “displacement” method. In the displacement method, a known volume of water is added to the graduated cylinder and this volume is recorded. Then the solid object is carefully placed in the cylinder and the new volume of the water + object is recorded. When these two volumes are subtracted, the volume of the solid object can be determined. As before, we always estimate in between the smallest increments marked on the cylinder. (Note: For some of the cylinders, the last increments marked are actually every 0.2 milliliters, so you'd have to estimate (for example) if it is 0.20, 0.25, 0.30, or 0.35 milliliters. This can be confusing, so ask if you need to.) To avoid any parallax, we must always measure the volume of water by viewing it from the exact same level as the water. Lastly, we always measure the volume at the bottom of the “meniscus” (a word that is on your next test) as seen in the figure below. 5. Place about 50 mL of water in a 100 mL graduated cylinder. Measure this volume, remembering to view and read the bottom of the meniscus at eye level, and to estimate the last digit in between the smallest increments on the graduated cylinder. Record this volume in Table B. 6. Add the 40 pennies minted before 1982. CAUTION: Add the pennies carefully so that no water is splashed out of the cylinder. Record the exact volume of the water and pennies. 7. Repeat steps 5 and 6 two more times and record in Table B. 8. Determine the volume of the pennies for each trial and record in Table B. 9. Average the results of those trials to determine the average volume of the pennies and record in Table B. C. MEASURING TEMPERATURE 1. Temperature is a quantitative measurement of how hot or cold something is. It is always measured in Celsius. A thermometer is a standard apparatus used for measuring temperature. Examine a thermometer. If it has both Celsius and Fahrenheit, you will have to be exceptionally careful that you are reading in Celsius. As with the other measuring devices, you must make sure that you are estimating a final digit in between the smallest increments on the thermometer. Since the increments on your thermometers are every one degree, you should be estimating every 0.1 degree. 2. Fill one beaker with water from the faucet and one with some ice water. (Ice is in the cooler in the front of the classroom. Use a lot!) Your hot water is the coffee urn. Measure the temperature in Celsius of the ice water, faucet water, and hot water, remembering to estimate the last digit in between the smallest increments on the thermometer. Record this data in Table C. 3. The class behind you will need to use your lab station and pennies. CLEAN UP YOUR STATION COMPLETELY and DRY YOUR PENNIES! Thank you! Mrs. Morgan appreciates your help!