Mass & Volume of a Pure Substance
advertisement

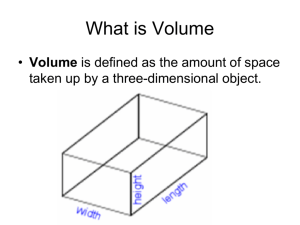
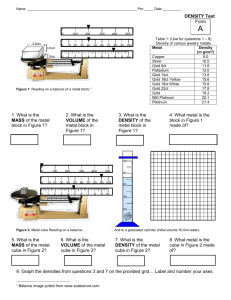
Name ___________________________ Date ________ Chemistry Mass & Volume of a Pure Substance The purpose of this lab is to introduce you the laboratory procedures of determining the mass of random objects, using water displacement to find the volume of solid objects, and how to calculate the density of random objects. The following 4 parts contain a variety of laboratory-investigative techniques that will prove useful for future completion and success in this course. Part A: Density of a Solid ~ Cube 1. Obtain a cube that is 1 centimeter high, 1 centimeter wide, and 1 centimeter long. 2. Determine the mass of the cube via the electronic scale. 3. Determine the volume of the cube by measuring it. What formula did you use? 4. Determine the volume of the cube via Water Displacement. 5. Mathematically determine the Density of the cube twice: once using the measurement volume and again using the water displacement volume. 6. How do these 2 densities compare to each other? Part B: Determining the Density of a piece of metal You may choose 1 metal rod from the collection on the teacher’s lab desk. 1. Determine the mass of the metal cylinder via the electronic scale. 2. Determine the volume of the metal cylinder via Water Displacement. 3. Mathematically determine the Density of the metal cylinder. 4. Determine the Percent Error for the metal cylinder. Percent error = Accepted value - Experimental value X 100 % Accepted value Part C: Determining the Density of a metal: 1. Using the metals provided, obtain 4 different size samples of the same metal. 2. Determine the mass, volume, and density of each clay sphere. Record your data in the following data table: Metal Type: 1 2 3 4 Mass (g) Volume (mL) Density (g/mL) Name ___________________________ Date ________ Chemistry 3. On graph paper, create a graph placing the volume on the x-axis and mass on the y-axis. 4. Use a ruler to draw the ‘best-fit’ straight line for the set of data points for each substance. Use your judgment to draw this line. Since measurements are estimates not all of the data points will fall on the line. Include the point (0,0). 5. Answer the following 2 questions: a. Calculate the average density of the metal using the slope of the line. b. What does this activity demonstrate about the relationship between the mass and the volume of a pure substance? Is density a characteristic (identifying) property of a substance? Part D: Density of Liquids 1. Using the clear liquid, obtain the mass of 10.0 mL, 20.0 mL, 30.0 mL, and 40.0 mL of the liquid, and do not subtract the mass of the graduated cylinder. 2. Record all measurements in the table provided. Sample Mass (g) Volume (mL) Density (g/mL) Liquid: #1 #2 #3 #4 3. 10.0 20.0 30.0 40.0 Repeat the process for the colored liquid. Sample Type: Mass (g) w/ graduated cylinder #1 #2 #3 #4 4. Volume (mL) Density (g/mL) 10.0 20.0 30.0 40.0 Graph the data for each of the liquids with mass on the y-axis and volume on the x-axis. Determine the slope of each line and compare to the average density of each liquid. Do not include the point (0,0) or subtract the mass of the graduated cylinder from the sample. Part E : Mass 1. Determine the mass of a beaker of pennies. Dump the pennies onto the lab table and measure the mass of the empty beaker. Subtract to determine the mass of all of the pennies. 2. Measure the mass of one penny. By dividing the total mass of the pennies by the mass of one penny, CALCULATE the number of pennies that were in the beaker. 3. Count the number of pennies in your beaker. Name ___________________________ Date ________ F. Chemistry a. Does the counted number agree with your calculated value? Explain. b. What assumption(s) were made in calculating the number of pennies? c. How could you improve the accuracy of the calculated number of pennies? d. Which numbers are measured, which are calculated; which numbers are estimated (notexact)? Are any of these numbers exact? a) Record the mass of an empty 100.0 mL graduated cylinder. Place 50.0 mL of water in a graduated cylinder and record its mass. Determine the mass of 50.0 mL of water. b) Count the number of drops of water in 1.0 mL. Using this data, determine the number of drops in 50.0 mL of water. c) Determine the mass of 1 drop of water.