J Dent Res 82(7): 523-527, 2003
advertisement

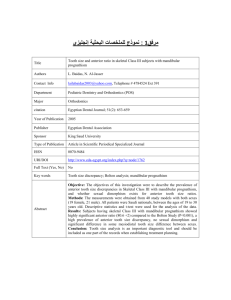
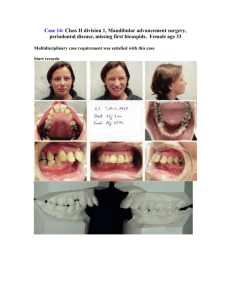
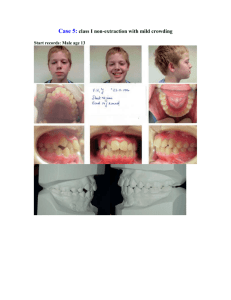
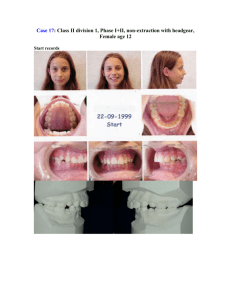
J Dent Res 82(7): 523-527, 2003 © 2003 International and American Associations for Dental Research RESEARCH REPORT Clinical Segregation Analysis of Mandibular Prognathism in Libya A.A. El-Gheriani1,2, B.S. Maher2, A.S. El-Gheriani3, J.J. Sciote4, F.A. Abushahba5, R. Al-Azemi4,7, and M.L. Marazita1,2,6,* 1 Department of Human Genetics, Graduate School of Public Health, 500 Cellomics Bldg., University of Pittsburgh, 100 Technology Dr., Pittsburgh, PA 15219; 2 Center for Craniofacial and Dental Genetics, Division of Oral Biology, School of Dental Medicine, University of Pittsburgh, PA; 3 Department of Prosthodontics, Faculty of Dentistry, Benghazi, Libya (currently Department of Restorative Dentistry, Ajman University of Science and Technology, Ajman, UAE); 4 Department of Orthodontics, School of Dental Medicine, University of Pittsburgh, PA 7 (currently Department of Orthodontics, Ministry of Health, Kuwait); 5 Department of Orthodontics, Faculty of Dentistry, Benghazi, Libya; and 6 Department of Oral and Maxillofacial Surgery, School of Dental Medicine, University of Pittsburgh, PA; *corresponding author, marazita@sdmgenetics.pitt.edu ABSTRACT The etiology of mandibular prognathism has been attributed to various genetic inheritance patterns and some environmental factors. The variation in inheritance patterns can be partly due to the use of different statistical approaches in the respective studies. The objective of this study was to investigate the role of genetic influences in the etiology of this trait. We performed segregation analysis on 37 families of patients currently being treated for mandibular prognathism. Mandibular prognathism was treated as a qualitative trait, with cephalometric radiographs, dental models, and photographs used to verify diagnosis. Segregation analysis of a prognathic mandible in the entire dataset supported a transmissible Mendelian major effect, with a dominant mode of inheritance determined to be the most parsimonious. KEY WORDS: mandible • mandibular prognathism • Class III malocclusion • genetics INTRODUCTION Mandibular prognathism is a common finding, with prevalence varying by race (Singh, 1999)—in particular, with higher prevalence in East Asians (Allwright and Bundred, 1964), Africans (Garner and Butt, 1985), and Caucasians (Emrich et al., 1965), respectively—and also varying by age, ranging from an approximate prevalence of 0.5% in children 6-14 yrs old (Newman, 1956) to a range of 2-4% in adults (Jorgenson, 1990). Despite many years of investigation, the relative contributions of genetic and environmental components in the etiology of non-syndromic mandibular prognathism are unclear. Mossey (1999) states that this is due to a lack of research dedicated to this problem, relatively imprecise measuring tools and limited knowledge about the genetic mechanisms involved, and the precise nature and effects of environmental influences. It should also be added that little is known about the interaction between genetic and environmental factors in the causation of mandibular prognathism. Mandibular prognathism cases have been associated with various environmental etiologies, such as: enlarged tonsils (Angle, 1907); endocrine imbalances (Downs, 1928); posture, trauma, and disease (Gold, 1949); hormonal disturbance (Pascoe et al., 1960); congenital anatomic defects (Monteleone and Duvigneaud, 1963); and instrument deliveries (Schoenwetter, 1974). Familial aggregation of mandibular prognathism has also been described and ascribed to a variety of genetic models, including autosomal-recessive (Downs, 1928; Iwagaki, 1938), autosomal-dominant (Stiles and Luke, 1953; Wolff et al., 1993), and a polygenic model of transmission (Litton et al., 1970). However, Kraus et al.(1959) maintained that "the role of heredity could not be discerned". Although the genetic models differ, there is a consensus that there is a role for genetics in determining the occurrence of mandibular prognathism. Understanding the specific genetic factors contributing to variation in the risk for mandibular prognathism would be a major advance in dentofacial orthopedics and oral and maxillofacial surgery. The goal of the current study was to apply modern methods of segregation analysis to examine specific genetic models of the familial transmission of mandibular prognathism in a series of large Libyan families. MATERIALS & METHODS Subjects The authors identified 37 probands with mandibular prognathism from the patient base of several dental clinics in Benghazi, Libya. Complete family histories for each proband and the affection status of other individuals in each family were confirmed by cephalometric, photographic, and/or dental models. The study sample of 37 families, comprised of 1013 individuals (285 first-degree relatives, 658 extended relatives, and 33 individuals related to the proband through marriage), is summarized in Table 1 . Consenting probands (or their parent/guardian) were asked to discuss this study with other family members prior to participation in the study. The study protocol was approved by the University of Pittsburgh Institutional Review Board, and informed consent was obtained from all subjects. View this table: [in this window] [in a new window] Table 1. Numbers of Individuals by Phenotype and Sex in 37 Families Ascertained through Probands with Mandibular Prognathism Procedure Assessment We determined mandibular prognathism by assessing one or more of the following orthodontic records: a lateral cephalogram, orthodontic study models, and/or lateral profile facial photographs (Fig. ). This assessment was done by Drs. A.S. El-Gheriani and F.A. Abu-shahba. The highest level of evidence was considered to be the radiographic records; if radiographic records were not available, then dental models were used. If no dental models were available, photographs were utilized. Cases for which affection status consensus could not be reached were classified as unknown. Figure. Shown in three different subjects are the diagnostic criteria for inclusion, either (a) a cephalogram, (b) a study model, or (c) a lateral photograph. View larger version (114K): [in this window] [in a new window] All 37 probands had a lateral cephalometric radiograph as part of their treatment record, and a confirmed negative ANB angle was a prerequisite for enrollment in the study; however, we were able to obtain lateral cephalometric radiographs on 11 subjects for further evaluation at the University of Pittsburgh School of Dental Medicine. These cephalograms were measured and compared with selected linear and angular values found in the Bolton Growth Study (Broadbent et al., 1975) (Table 2 ). Since the data are stratified by age and sex, we chose pooled sex measurements at age 12 as the mean value for each measurement for comparative purposes. Although interand intra-rater reliability measurements were not assessed, we used the 11 cephalographs as confirmation of the clinical diagnosis made on all subjects, since the cephalometric values confirmed a skeletal class III diagnosis in all cases. View this table: Table 2. Cephalometric Analysis Results [in this window] [in a new window] This criterion establishes the forward relationship of the mandible to the maxilla. Affection status of relatives (on whom radiographic records were not available) was determined by the presence of an edge-to-edge incisor relationship or an anterior crossbite in dental casts articulated in centric jaw relation. The final diagnostic tool was the utilization of lateral photographs on individuals for whom radiographic and dental cast records were unavailable. The subject was determined to have a prognathic appearance (concave facial profile) when viewed in a lateral photograph. Individuals for whom these records were not available were classified as unknown. Statistical methods We investigated the role of genetics and other influences in the skeletal malocclusion family patterns by fitting class A regressive models (Bonney, 1984) as implemented by the REGD routine (for dichotomous traits) in S.A.G.E. release 3.1 [SAGE, 1994]. These models assume that variation in a trait among individuals results from a major gene effect and residual variation which may reflect both familial correlations and individual variation. The class A models assumed that the similarity between siblings is due only to common parentage. We tested hypotheses by fitting a more complete model and comparing the resulting likelihood with those of reduced nested models. The analysis model allowed for statistical detection of major transmissible effects in a dichotomous trait, but did not allow for explicit tests of multigenic/multifactorial contributions to the trait. Such tests are not readily available for analysis of a dichotomous trait in extended kindreds. The hypothesis of most interest was whether there was a major effect that would then allow the application of the powerful genemapping methods to identify specific gene(s) involved in mandibular prognathism. Such gene-mapping methods will also allow for assessment of multigene contributions to the trait, and also gene x environmental interactions. The overall model that was used allows for two alleles (A and B) at a single locus, resulting in three genotypes or "types" (AA, AB, BB). For our analyses, we assumed the distribution of the three types in the population to be in Hardy-Weinberg equilibrium. In the "no transmission" test, there is only one type. The parameters of the models include the regression coefficients for each "type" (ßAA, ßAB, ßBB), and the frequency of allele A (denoted qA). Individuals of types AA, AB, and BB were assumed to transmit the A allele to their offspring with probabilities AA, AB, and BB. These transmission probabilities were used to calculate the probabilities of all three types for individuals whose parents are in the pedigree. The segregation analyses consisted of fitting a series of models ranging from simple no-transmission models to the most general transmission model. The general model was fitted and compared with various more restricted submodels. To guard against the presence of multiple local maxima, we used several initial estimates of the parameters. Models that were tested for skeletal malocclusion will include no-transmission models, A-dominant, A-recessive (B-dominant), additive and co-dominant major gene models, and a general model. The no-transmission model assumed one type with all individuals being independent of one another. The A-dominant model restricted ßAA = ßAB, while a B-dominant model restricted ßAB = ßBB. The additive model assumed ßAB to be the average of ßAA and ßBB. The co-dominant major gene model put no restrictions on the types. Under these genetic models, the transmission parameters were restricted to the Mendelian expected values of AA = 1, AB = 0.5 and BB = 0. For us to utilize the general model in these analyses, there were no restrictions on the regression coefficients of the types or on tAB, allowing for tests of Mendelian transmission. Segregation analysis is sensitive to the method of identifying families, i.e., the ascertainment scheme used. In these studies, families were ascertained through probands. The analysis was therefore adjusted for the ascertaining scheme by conditioning on the probands when the likelihood calculations were performed. Hypotheses were tested according to the Likelihood Ratio Criterion, which is the ratio of the maximum value of the likelihood under the most general model to the likelihood under a restricted model. Each null hypothesis corresponds to one or more restrictions being placed on the most general model to the likelihood under a restricted model. The models were evaluated with a test statistic defined as minus the log of the likelihood ratio criterion. The distribution of this statistic was approximated by a 2 distribution with the number of degrees of freedom equal to the difference in the numbers of parameters between the model under the null hypothesis and the more general model. The null hypothesis was rejected if this statistic is greater than the 2 value corresponding to the desired significance level ( = 0.05). Additionally, equally likely models were assessed based on Akaike’s information criteria (AIC; Akaike, 1974). For any given model, the AIC = -2ln(L) + 2 number of parameters estimated. The model with the lowest AIC was considered to be the most parsimonious among equally likely models. RESULTS The segregation analysis results are summarized in Table 3 . Compared with the general model, the no-transmission model (p = 0.01) and co-dominant major gene model (p < 0.001) were both rejected. Autosomal-dominant, -recessive, and additive major locus models could not be rejected by the likelihood ratio test (all p-values > 0.14). Of the models that were not rejected, the autosomal-dominant major locus model was determined to be the most parsimonious (AIC = 531.65). View this table: Table 3. Results of Segregation Analysis [in this window] [in a new window] DISCUSSION Our results support the previous findings that there is a hereditary component to the expression of this phenotype. We were able to rule out the "no transmission" and codominant models. By utilizing the AIC, we were able to conclude that, among the autosomal-dominant, -recessive, and additive models, the autosomal-dominant model was the most parsimonious. Our conclusion of autosomal-dominant inheritance was in agreement with Wolff et al.(1993), who utilized pictures or authentic descriptions to determine affection status, but disagreed with the polygenic conclusion of Litton et al.(1970). Note that Litton et al.(1970) relied on articulated dental casts, while we used cephalograms as a means of determining affection status. Now that we have statistical genetic evidence for major locus involvement in mandibular prognathism, gene-mapping studies will be feasible. Gene-mapping studies would have the potential to identify the gene or genes involved in the trait, providing powerful confirmation for a genetic basis for mandibular prognathism. Such studies will also allow for tests of multiple genes to detect any etiological heterogeneity in the trait. Careful clinical characterization of mandibular prognathism will be key to additional progress in our understanding of the genetics of this common disorder. The Steiner analysis is readily known and easy to perform, and, as Saunders et al.(1980) reported, there is a positive correlation between the ANB angle among family members. Note, however, that the important points are the relative position of the mandible to the cranial base/maxilla, and also which aspects of the skeletal morphology seem to be associated with the relative mandibular prognathism. The drawback to basing this study on cephalometric records was the ethical implication of unnecessary radiation exposure. Therefore, the probands who were required to have radiographs taken as part of their routine treatment were confirmed radiographically; however, the affection status of other relatives could not be verified unless they were also under orthodontic treatment. The use of dental models, as proposed by Ngan et al.(1997), to diagnose Class III malocclusion overcomes the radiation exposure issue; however, the contributions of each growth site and anatomic area cannot be determined in the overall phenotypic expression when diagnosis is based on dental models. The cephalometric analysis (Table 2 ) highlights the difficulty in the study of this trait. The ages ranged from 10 to 38 yrs, and the ANB angles ranged from -0.9° to 9.1°. Even though all 11 subjects have a negative ANB, there were noticeable differences in their cephalometric measures that warrant the notion that there may be multiple factors or multiple types of mandibular prognathism (Jacobson et al., 1974). The Bolton standards were compared with our measured values because there are no definitive cephalometric norms for the Libyan population, and Libyan schoolchildren have been shown to have a cephalic index similar to that of European schoolchildren (Gardiner, 1982). Other than the fact that the phenotype is difficult to define correctly, craniofacial growth, and particularly the growth of the mandible, is highly variable and is reported to continue into the late teens and well beyond the third decade of life (Behrents, 1985), although Tollaro et al.(1994) reported that a distinctive Class III pattern could be detected in children with complete deciduous dentitions (age 4-6 yrs). It is not reasonable for clinicians to delay treatment for those individuals exhibiting mandibular prognathism in an attempt to see the final phenotype, and conversely, could one compare the untreated cases of mandibular prognathism (individuals in their late 20s and beyond) with those of pre-pubescent children who are still growing? Litton et al.(1970) used a cut-off point of 15 years of age, such that all unaffected children below the age of 15 were classified as unknown, since there is potential for them to develop this condition at a later time. We did not incorporate such a condition into our study and will be looking at the incidence of mandibular prognathism with increasing age for future studies. The emphasis now should be placed on devising a safe and acceptable method of not only diagnosing mandibular prognathism, but also investigating the inheritance patterns of each skeletal morphologic characteristic that may contribute to it. Once a definitive method of phenotype classification is developed, this study will be expanded to other population groups in the US, Europe, Africa, and the Middle East. Finally, given this statistical evidence of a major transmissible effect in mandibular prognathism, we will begin linkage studies to identify the specific gene(s) involved in the trait. ACKNOWLEDGMENTS This study was supported by NIH grant T35-DE07336. The results of this paper were obtained by use of the program package S.A.G.E., which is supported by a US Public Health Service Resource Grant (1 P41 RR03655) from the National Center for Research Resources. We also thank Drs. M.K. Nair, F. Hamad, A. Sultan, R.E. Ferrell, M. Rahouma, H. Ben Khayal, Mr. F Reybod, and the study subjects for their contributions to this research. Received September 5, 2002; Last revision January 13, 2003; Accepted April 15, 2003 REFERENCES Akaike H (1974). A new look at the statistical model identification. IEEE Trans Automatic Control AC 19:616–623. Allwright WC, Bundred WH (1964). A survey of handicapping dentofacial anomalies among Chinese in Hong kong. Int Dent J 14:505–519. Angle EH (1907). Treatment of malocclusion of the teeth. 7th ed. Philadelphia: S.S. White Manufacturing Company. Behrents RG (1985). The biological basis for understanding craniofacial growth during adulthood. Prog Clin Biol Res 187:307–319.[Medline] Bonney GE (1984). On the statistical determination of major gene mechanisms in continuous human traits: regressive models. Am J Med Genet 18:731– 749.[ISI][Medline] Broadbent BH Sr, Broadbent BH Jr, Golden WH (1975). Bolton standards of dentofacial growth. St. Louis: The C.V. Mosby Company. Downs WG (1928). Studies in the causes of dental anomalies. J Dent Res 28:267– 379, Emrich RE, Brodie AG, Blayney JR (1965). Prevalence of class I, class II, and class III malocclusions (Angle) in an urban population; an epidemiological study. J Dent Res 44:947–953.[Abstract/Free Full Text] Gardiner JH (1982). An orthodontic survey of Libyan schoolchildren. Br J Orthod 9:59–61.[Medline] Garner LD, Butt MH (1985). Malocclusion in black American and Nyeri Kenyans. An epidemiologic study. Angle Orthod 55:139–146.[ISI][Medline] Gold JK (1949). A new approach to the treatment of mandibular prognathism. Am J Orthod 35:893–912. Iwagaki H (1938). Hereditary influence of malocclusion. Am J Orthod Oral Surg 24:328–336. Jacobson A, Evans WG, Preston CB, Sadowsky PL (1974). Mandibular prognathism. Am J Orthod 66:140–171.[ISI][Medline] Jorgenson RJ (1990). Mandibular prognathism. In: Birth defects encyclopedia. Buyse ML, editor. Cambridge: Blackwell Scientific Publications. Kraus BS, Wise WJ, Frie RH (1959). Heredity and the craniofacial complex. Am J Orthod 45:172–217. Litton SF, Ackermann LV, Isaacson RJ, Shapiro BL (1970). A genetic study of class III malocclusion. Am J Orthod 58:565–577.[ISI][Medline] Monteleone L, Duvigneaud JD (1963). Prognathism. J Oral Surg 21:190–195. Mossey PA (1999). The heritability of malocclusion: Part 2. The influence of genetics in malocclusion. Br J Orthod 26:195–203.[Abstract/Free Full Text] Newman GV (1956). Prevalence of malocclusion in children six to fourteen years of age and treatment in preventable cases. J Am Dent Assoc 52:566–575. Ngan P, Hu AM, Fields HW Jr (1997). Treatment of class III problems begins with differential diagnosis of anterior crossbites. Pediatr Dent 19:386–395.[Medline] Pascoe J, Hayward JR, Costich ER (1960). Mandibular prognathism, its etiology and a classification. J Oral Surg 18:21–24.[Medline] SAGE (1994). Statistical analysis for genetic epidemiology. Rel. 2.2. Computer program package available from the Department of Epidemiology and Biostatistics, Case Western Reserve University, Cleveland, OH. Saunders SR, Popovich F, Thompson GW (1980). A family study of craniofacial dimensions in the Burlington Growth Centre sample. Am J Orthod 78:394– 403.[ISI][Medline] Schoenwetter RF (1974). A possible relationship between certain malocclusions and difficult or instrument deliveries. Angle Orthod 44:336–340.[ISI][Medline] Singh GD (1999). Morphologic determinants in the etiology of class III malocclusions: a review. Clin Anat 12:382–405.[ISI][Medline] Stiles KA, Luke JE (1953). The inheritance of malocclusion due to mandibular prognathism. J Hered 44:241–245.[Free Full Text] Tollaro I, Baccetti T, Bassarelli V, Franchi L (1994). Class III malocclusion in the deciduous dentition: a morphological and correlation study. Eur J Orthod 16:401– 408.[Abstract/Free Full Text] Wolff G, Wienker TF, Sander H (1993). On the genetics of mandibular prognathism: analysis of large European noble families. J Med Genet 30:112–116.[Abstract]