MISE - Physical Basis of Chemistry
advertisement

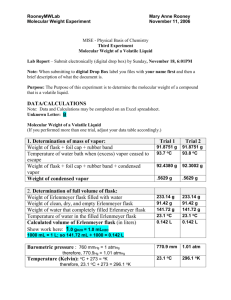
WagenborgMWVOLGASLab Page 1 Name:Bill Wagenborg MISE - Physical Basis of Chemistry Third Experiment Molecular Weight of a Volatile Liquid Lab Report – Submit electronically (digital drop box) by Sunday, November 18, 6:01PM Note: When submitting to digital Drop Box label you files with your name first and then a brief description of what the document is. Purpose: The Purpose of this experiment is to determine the molecular weight of a compound that is a volatile liquid. DATA/CALCULATIONS Note: Data and Calculations may be completed on an Excel spreadsheet. Unknown Letter: ____C_________ Molecular Weight of a Volatile Liquid (If you performed more than one trial, adjust your data table accordingly.) 1. Determination of mass of vapor: Weight of flask + foil cap + rubber band Trial 1 81.3358 g Temperature of water bath when (excess) vapor ceased to escape Weight of flask + foil cap + rubber band + condensed vapor Weight of condensed vapor 89 0 C 2. Determination of full volume of flask: Weight of Erlenmeyer flask filled with water Weight of clean, dry, and empty Erlenmeyer flask Weight of water that completely filled Erlenmeyer flask Temperature of water in the filled Erlenmeyer flask Calculated volume of Erlenmeyer flask (in liters) Show work here:Density of water at 21 C = .997992 D=M/V V=M/D = 146.7/.997992= 80.8894 g .4464 g 227.8 g 81.1 g 146.7 g 21 0 C .147 L Trial 2 81.3413 g 90 0 C 80.9104 g .4309 g WagenborgMWVOLGASLab Page 2 146.995 ml (146.995 ml)(1 L/ 1000ml) =.1469951663 L Barometric pressure : Temperature (Kelvin): 770.9mmHg(1.01Atm) 294 0 K Determination of molecular weight of the volatile liquid: (Assuming ideal gas behavior, use the pressure of the vapor (P), its volume (V), and its temperature (T) to determine the moles of vapor in the Erlenmeyer flask. Then, using the mass of vapor which occupied the Erlenmeyer flask, determine the molecular weight (MW in g/mole) of the volatile liquid. If you performed more than one trial, show all work for each trial and then compute the average molecular weight.) Show work below. PV=NRT N= PV/RT N= (1.01)(.147)/(.0821)(362) = .0049955922 MOLES MW(1) = MW(2)= gRT (.4464)(.0821)(362) 89.60562215 g / mole PV (101 . )(.147) gRT (.4309)(.0821)(363) 86.49431582 g / mole PV (101 . )(.147) (89.60562215 + 86.49431582) / 2 = 88.04996899 (Average) Molecular Weight 88.05 g/mole Determination of Empirical and Molecular Formula of volatile liquid: The table below lists the pure volatile liquids that were distributed in lab. The first column gives the letter designation of the compound and the remaining columns give the appropriate elemental mass percents. Using the data for your particular volatile liquid, please determine the empirical formula and the molecular formula for your particular sample. Show all work. Sample Letter mass % carbon (C) mass % hydrogen (H) mass % oxygen (O) “A” (cyclohexane) 85.60 % 14.40 % None “B” (ethyl acetate) 54.52 % 9.17 % 36.31 % “C” (2-propanol) 59.94 % 13.44 % 26.62 % 1mole 4.99 moles of C 12.01 g 1mole 13.31moles of H Moles Of H = (13.44 g) 101 . g Moles of C = (59.94 g) WagenborgMWVOLGASLab Page 3 Moles of oxygen = (26.62 g) 1mole 166 . moles O 16 g Moles C/ moles O = 4.99/1.66 = 3 Moles H/moles O = 13.31 / 1.66 = 8 MW/EFW = N EFW = (12.01 * 3) + (1.01 * 8) + (16 *1) =60.11 88.05/60.11 = 1.46= 1 C = 1 *3 = 3 H=1 * 8 = 8 O= 1 * 1 = 1 (1 * THE EMPIRICAL FORMULA) Empirical Formula C3 H 8 O Molecular Formula C3 H8 O CONCLUSION QUESTIONS 1.Why is the barometric (i.e., atmospheric) pressure considered to be the pressure of the vapor, i.e., how does the experimental procedure ensure this? Explain carefully. We know that the pressure on the outside (barometric pressure) is equal to the pressure on the inside (the vapor) because we do not see any vapor escaping through the pinhole after awhile. For this to occur, both pressures must be equal. This experimental procedure points to the pressure being equal. 2. Why isn't it necessary to weigh the amount of liquid initially put into the flask? Explain. We do not have to weigh the amount of liquid in the flask because it ( the liquid’s weight) does not play play a part in the final outcome. During the heating excess vapor escaped through the pinhole. The weight of the vapor in the flask after the evaporation is what we are concerned with because that is crucial to our calculations. We need that along with the pressure, temperature and the volume of the gas to plug into the ideal Gas formula in order to find the molecular weight. Since we are using the barometric pressure we need to use the weight of the vapor when the vapor’s pressure matches it (the barometric pressure). Extra Credit– It is sometimes stated that the above method (Dumas method) relies on the presumption that the investigated gas follows the ideal gas law (PV = nRT). This allows one to determine the moles of the contained vapor via: PV n = moles of contained vapor = RT , i.e., its measured pressure (P), volume (V), and temperature (T). Of course, the gas constant (R) is tabulated and equals 0.0821 L• atm•mol-1•K-1. Then, the molecular weight of the gas (MW in g/mol) can be determined from the measured mass of the contained vapor (g in grams) divided WagenborgMWVOLGASLab Page 4 by the calculated number of moles. g g gRT MW = = = . In other words, if the gas did not behave ideally, then the n PV PV RT calculated molecular weight would be a crude approximation at best. Please explain why this is commonly not a big problem, i.e., why an approximate value of the molecular weight is often sufficient when determining the actual chemical formula of a volatile compound. What other information is commonly obtained for a compound in the process of determining its chemical formula such that an approximate molecular weight is often “good enough”? It is good enough because along with the molecular weight, we use the empirical formula weight to find the chemical (molecular formula). We divide the molecular weight by the empirical formula weight to find an integer to multiply the empirical formula by. If the molecular weight is not exact, it does not matter because we will round the answer we get to a whole number to multiply the empirical formula by. In the end the chemical formula will be correct.