Dumas - Cameron University
advertisement

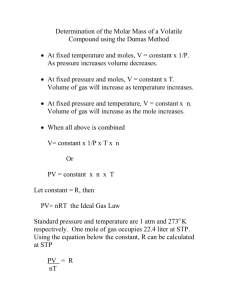
For best results please view this as a slide show. You can hit the F5 key or go to the Slide Show tab on the menu bar and click on From Beginning. Page Down and Page Up will move you through the presentation. If you have a Mac do whatever you have to do to play it as a slide show – I don’t know Macs well. Dr. Buckley e-mail: gbuckley@cameron.edu Experiment #15 – Determining the Molecular Weight of a Volatile Liquid by the Dumas Method Laboratory Overview CHEM 1361 August 2010 Gary S. Buckley, Ph.D. Department of Physical Sciences Cameron University Table of Contents (you may click on any of the topics below to go directly to that topic) •Experimental Objectives •Ideal Gas Law •Experimental Procedure Experimental Objectives •Acquire experimental data related to the ideal gas law and solve for missing quantities •Become acquainted with the Dumas method for determining the molar mass of a volatile liquid •Find the molar mass of a volatile substance from its volume, temperature, and pressure using the ideal gas law Ideal Gas Law The ideal gas law expresses the mathematical relationship between the pressure, volume, temperature, and number moles of an ideal gas. It may be given as: PV = nRT where P is the pressure, V the volume, n the number of moles of gas, R a constant, and T is the temperature. The units chosen for P and V must be consistent with the value used for R, the ideal gas constant. R has only a single value, but can be expressed in different sets of units (similar to length, for example, 1 yard = 3 feet = 36 inches, all of which represent the same length). A couple of typical methods for expressing R include: R 0.08206 L atm L torr 62.36 mol K mol K In applying the ideal gas equation, the units chosen for pressure, volume , and temperature must match those of the selected gas constant. More on Application of the Ideal Gas Law In this particular experiment, you will take measurements of the mass of the vapor of a sample, its pressure, volume, and temperature in order to determine the number of moles in a flask. Based on the mass and calculated value of n, the number of moles, the molar mass can be determined. From the ideal gas law PV n RT so experimental determination of P, V, and T will give the number of moles of gas in a container. By measuring the mass of the gas the molar mass may be determined from molar mass = mass of substance mol of substance Experimental Procedure In the Dumas method, a volatile liquid is placed in a dry flask. A piece of aluminum foil with a pinhole is placed over the opening in the flask and the apparatus placed in boiling water. The liquid will vaporize slowly through the pinhole until it is gone from the flask. At the point at which the last bit of liquid disappears, the flask is filled only with the vapor of the volatile liquid. Once the flask is removed from the boiling water, the will condense and its mass may be determined. Al foil cap with pin hole The temperature is assumed to be that of boiling water, the volume of the flask is determined by measuring the mass of water required to completely fill the flask, and the pressure is atmospheric pressure determined from a barometer in the room. From application of the ideal gas law the number of moles of condensate – effectively the mass of vapor in the flask - may be determined. The trickiest part of this experiment is determining when the last drop of liquid has vaporized. If you quit boiling too soon you will have liquid in the flask that will be counted as condensate; if too late you will lose some of the condensate through vaporization.