Gases Review
advertisement

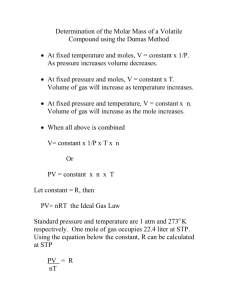
Gases Review 1. Write mathematical expressions for the following gas laws. You should define all symbols and state any constant conditions. (a) Boyle’s PV = a constant at constant T and n (b) Charles’s V/T = a constant at constant P and n (c) Avogadro’s V/n = a constant at constant T and P (d) Gay-Lussac’s P/T = a constant at constant V and n (e) Combined P V/T = a constant at constant n (f) Ideal P V = n R T 2. In gas law problems, the temperature should always be in what unit? Kelvins 3. What pressure, in torr, would a sample of Xenon exert if it were compressed from 4001 mL to 3002 mL at constant temperature, given that initially the pressure was 750.1 atm? 7.60 x 105 torr 4. An almost empty aerosol can has an internal pressure of 1.030 atm when the temperature is 25.00 °C. What would be the pressure in the can if it were placed into an incinerator for disposal, which would have the effect of raising the temperature inside the can to 1500.°C? Why is the incineration of aerosol cans not recommended? 6.13 atm, the can could explode 5. In an industrial process, 200. L of a gas at 91.0 kPa and 21.0 °C is compressed into a vessel that has a volume of 15.2 L and heated to 420. °C. What is its final pressure? 2822 KPa 6. Calculate the pressure inside a tube, given that the tube’s volume is 5.0 L, its temperature is 23 °C, and it contains 0.010 mg of hydrogen gas. 2.43 x 10-5 atm 7. Calculate the molar volume (volume of 1.0 mole) of an ideal gas at 1.0 atm and 25 °C? 24.45 L 8. Re-write the ideal gas equation to make molar mass the subject of the equation. Molar mass = (mass)(R)(T) (P) (V) 9. In a given period of time, 0.21 moles of a gas of molar mass = 26 g/mol effuses. How many moles of NH3 would effuse in the same period of time? 0.26 moles 10. This questions deals with gas mixtures. (a) Write an equation for total pressure of a mixture of three gases, A, B, and C, in terms of the partial pressures of the gases. Ptotal = PA + PB + PC (b) Write an equation for the total pressure of a mixture of three gases in terms of the total number of moles of gas. Ptotal = ntotal (R)(T) (V) 11. Small quantities of hydrogen gas can be prepared in the laboratory by the following reaction: Zn(s) + 2 HCl(aq) ZnCl2(aq) + H2(g) In such an experiment, 454 mL of hydrogen gas were collected over water. The gas mixture includes hydrogen and water vapor. The temperature of this gas mixture was 23.0 °C and the total pressure was 712 mm Hg. How many moles of hydrogen did you prepare? The vapor pressure of water at 23.0°C is 19.8 mm Hg. 0.017 moles 12. Calculate the molar mass of a vapor that would effuse at a rate equal to 70.6 % of the rate of a gas with a molar mass = 17.0 g/mol. 34.1 g/mol 13. Calculate and compare the urms of Radon gas at 35.0 oC and 299 K. 186 m/s, 183 m/s 14. What are the key points to the Kinetic Molecular Theory? The volume of the gas particles is insignificant compared to the volume of the container Gas particles move randomly, in all directions, in straight lines Forces of attraction or repulsion between the particles is weak and insignificant When particles collide with each other the average kinetic energy for the container is unchanged The average kinetic energy of a particle is proportional to the Kelvin temperature 15. When will gases tend to deviate from behaving ideally? High pressure and low temperature 16. Equal masses (0.500 g each) of hydrogen and oxygen are placed in an evacuated 4.00 L flask at 25.0°C. The mixture is allowed to react to completion and the flask is returned to 25.0°C and allowed to come to equilibrium. The equilibrium vapor pressure of water at 25°C is 23.76 torr. a. b. c. d. e. f. Write and balance an equation for the reaction. 2H2 (g) + O2 (g) 2H2O What is the total pressure inside the flask before the reaction begins? 1.62 atm What is the mass of water vapor in the flask at equilibrium? 0.092 g H2O vapor Which reactant gas is limiting? O2 How many grams of the excess reactant remain at equilibrium? 0.438 g H2 remain What is the total pressure inside the flask at equilibrium? 1.37 atm