MISE - Physical Basis of Chemistry
advertisement

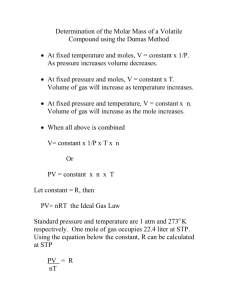
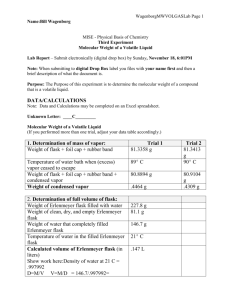
Page 1 NAMES: Mary-Kate McGlinchey MISE - Physical Basis of Chemistry Third Experiment Molecular Weight of a Volatile Liquid Lab Report – Submit electronically (digital drop box) by Sunday, November 6, 6:01PM Note: When submitting to digital Drop Box label you files with your name first and then a brief description of what the document is. Purpose: The Purpose of this experiment is to determine the molecular weight of a compound that is a volatile liquid. By vaporizing a measured amount of the sample, and determining the pressure, volume, and temperature of the sample, the approximate molecular weight may be determined by presuming ideal gas behavior. Background: • # 2 - Pressure, Gas Laws, Ideal Gas Law, Partial Pressures - Some Examples • Gases - Experiments and Relationships - PowerPoint Summary (PowerPoint summary of our discussion of gases.) These handouts are on Bb. Reference: For some procedural aspects of this lab: Catalyst - The Prentice-Hall Custom Laboratory Program for Chem 53 at the University of Pennsylvania Department of Chemistry, Pearson Custom Publishing, 2005, pages 57-65. Introduction: In Experiment # 2, you had the opportunity to determine the empirical formula of a binary compound. In order to determine the molecular formula of a compound - given the empirical formula - you need the molecular weight of the compound (in addition to the empirical formula weight). In this experiment, you will utilize one of many experimental procedures to determine the molecular weight of a compound. This method, first developed by Dumas, exploits the ideal gas law in order to determine the molecular weight of a compound that can be vaporized (without significant decomposition). Specifically - via the ideal gas law - you will determine the molecular weight of a substance by determining the pressure exerted, volume occupied, temperature, and mass of a sample of its vapor. The example below will hopefully enable you to understand how this is accomplished. Presuming that the vaporized volatile liquid follows the ideal gas law (PV = nRT), we may determine the moles of the contained vapor via: Page 2 PV n = moles of contained vapor = RT , i.e., its measured pressure (P), volume (V), and temperature (T). Of course, the gas constant (R) is tabulated and equals 0.0821 L• atm•mol-1•K-1. Then, the molecular weight of the gas (MW in g/mol) can be determined from the measured mass of the contained vapor (g in grams) divided by the calculated number of moles. g g gRT MW = n = PV = . PV RT Before you carry out the determination of the molecular weight (MW) of the volatile liquid, a worked example may help. Please refer to the Appendix at the end of this experiment. DATA/CALCULATIONS Note: Data and Calculations may be completed on an Excel spreadsheet. Unknown Letter: _______A______ Molecular Weight of a Volatile Liquid (If you performed more than one trial, adjust your data table accordingly.) Weight of flask + foil cap + rubber band 81.44 g Temperature of water bath when (excess) vapor ceased to escape 94.0 Weight of flask + foil cap + rubber band + condensed vapor Weight of condensed vapor 0.44 ºC 81.88 g g Determination of full volume of flask: Weight of Erlenmeyer flask filled with water 227.4 Weight of clean, dry, and empty Erlenmeyer flask g 80.98 g Weight of water that completely filled Erlenmeyer flask 146.42 Temperature of water in the filled Erlenmeyer flask 24 Calculated volume of Erlenmeyer flask _____0.147______ L Barometric pressure 752 mm Hg (xxx.x mm Hg) g ºC Page 3 Before you carry out the determination of the molecular weight (MW) of the volatile liquid, a worked example may help. Please refer to the Appendix at the end of this experiment. Table 1: (Revised**) Density of pure liquid water at various temperatures: Temperature (ºC) 1ºC 2ºC 3ºC 4ºC 5ºC 6ºC 7ºC 8ºC 9ºC 10ºC 11ºC 12ºC 13ºC 14ºC density (d) in g/mL 0.999890 0.999940 0.999960 0.999970 0.999970 0.999950 0.999910 0.999860 0.999790 0.999720 0.999620 0.999520 0.999400 0.999270 Temperature (ºC) 15ºC 16ºC 17ºC 18ºC 19ºC 20ºC 21ºC 22ºC 23ºC 24ºC 25ºC 26ºC 27ºC 28ºC density (d) in g/mL 0.999099 0.998943 0.998774 0.998595 0.998405 0.998203 0.997992 0.997770 0.997538 0.997296 0.997044 0.996783 0.996512 0.996232 ** Density data from 1ºC to 14ºC (at 0.1013 Mbar (1 atm)) source: NIST - National Institute of Standards & Technology website: http://webbook.nist.gov/ Determination of the volume occupied by the vapor in the Erlenmeyer flask: (Using the mass of water that completely filled the Erlenmeyer flask and the above table of water densities as needed – determine the volume (in L) occupied by the vapor in the Erlenmeyer flask. Also, explain your reasoning. Then, place your result in the data table at the appropriate spot. Show work below. Volume of vapor 0.147 L Determination of molecular weight of the volatile liquid: (Assuming ideal gas behavior, use the pressure of the vapor (P), its volume (V), and its temperature (T) to determine the moles of vapor in the Erlenmeyer flask. Page 4 Then, using the mass of vapor which occupied the Erlenmeyer flask, determine the molecular weight (MW in g/mole) of the volatile liquid. If you performed more than one trial, show all work for each trial and then compute the average molecular weight.) Show work below. (Average) Molecular Weight 90.41 g/mole Determination of Empirical and Molecular Formula of volatile liquid: The table below lists the pure volatile liquids that were distributed in lab. The first column gives the letter designation of the compound and the remaining columns give the appropriate elemental mass percents. Using the data for your particular volatile liquid, please determine the empirical formula and the molecular formula for your particular sample. Show all work. Sample Letter mass % carbon (C) mass % hydrogen (H) mass % oxygen (O) “A” (cyclohexane) 85.60 % 14.40 % None “B” (ethyl acetate) 54.52 % 9.17 % 36.31 % “C” (2-propanol) 59.94 % 13.44 % 26.62 % Page 5 Empirical Formula CH2 Molecular Formula C6H12 CONCLUSION QUESTIONS 1. Why is the barometric (i.e., atmospheric) pressure considered to be the pressure of the vapor, i.e., how does the experimental procedure ensure this? Explain carefully. Initially, the pressure inside the flask was greater than the barometric pressure which was outside the flask. As the vapor escaped, due to heating, both pressures equalized. The experiment ensured this due to the minute pinhole in the foil on top of the flask. This allowed substance “A’s” vapor to escape until there was no more vapor visible, which meant the pressures had equalized. Therefore, I could infer that the pressure inside the flask (the pressure of the vapor) could be considered the barometric pressure. 2. Why isn't it necessary to weigh the amount of liquid initially put into the flask? Explain. It isn’t necessary to measure the weight of the liquid that is initially put into the flask because some of the liquid will be changed into a vapor, which will escape through the pinhole. Therefore, for experimental purposes, some of the substance is lost when it changes from a liquid to a gas. Much of the liquid is vaporized throughout the experiment anyway. Extra – It is sometimes stated that the above method (Dumas method) relies on the presumption that the investigated gas follows the ideal gas law (PV = nRT). This allows one to determine the moles of the contained vapor via: PV n = moles of contained vapor = RT , i.e., its measured pressure (P), volume (V), and temperature (T). Of course, the gas constant (R) is tabulated and equals 0.0821 L• atm•mol-1•K-1. Then, the molecular weight of the gas (MW in g/mol) can be determined from the measured mass of the contained vapor (g in grams) divided by the calculated number of moles. g g gRT MW = n = PV = . In other words, if the gas did not behave ideally, then the PV RT calculated molecular weight would be a crude approximation at best. Please explain why this is commonly not a big problem, i.e., why an approximate value of the molecular weight is often sufficient when determining the actual chemical formula of a volatile compound. What other information is commonly obtained for a compound in the process of determining its chemical formula such that an approximate molecular weight is often “good enough”? Page 6 Appendix - A worked Example: A certain volatile hydrocarbon (a binary compound of carbon and hydrogen) is found to be 92.3 % carbon, by mass. In a separate experiment, utilizing the Dumas method, a 4.00 mL pure liquid sample of this hydrocarbon is vaporized in a 125 mL Erlenmeyer flask when the barometric pressure is 768.0 torr. The empty flask - fitted with a foil cap pierced with a pinhole - weighs 25.3478 g. After the excess gas escapes, the temperature is measured as 98.0oC. The flask and contents are subsequently cooled to 25oC and the vapor condenses to a liquid. The weight of the flask and contents is found to be 25.6803 g. The flask is then emptied, cleaned, and filled with water. When weighed on a triple-beam balance, the difference in weight between the flask filled to the brim with water and the dry empty flask – at 25ºC - is 128.12 g. Please determine the following: • The empirical formula of this hydrocarbon. • The volume that the vapor occupied in the Erlenmeyer flask (in L). • The molecular formula of this hydrocarbon. Solution to Example: The ideal gas law in terms of mass (g = grams), molecular weight (MW), temperature (T), volume (V), and pressure (P). mass g PV = nRT and n = moles = molecular weight = . MW g PV gRT n = MW = RT Solving for MW: MW = . PV The first equation above may be easily used to find the molecular weight of the L atm. hydrocarbon. Note that the ideal gas constant R = 0.0821 mole K . Also note that 760 torr = 1 atm. The kelvin (K) temperature is obtained by adding 273 to the temperature in oC. The first part of this problem, obtaining the empirical formula. A hydrocarbon contains only carbon and hydrogen. Thus, the mass % of H = 100 % - 92.3 % = 7.70 % H. We assume 100 g of the hydrocarbon. Then, there are 92.3 g of C and 7.70 g of H present (relatively). Determining moles of each element: 1 mole C moles C = (92.3 g C)•12.01 g C = 7.69 moles C. moles H = 1 mole H (7.70 g H)• 1.01 g H Divide to obtain the mole ratio: = 7.62 moles H. Page 7 moles C 7.69 moles 1.01 1 moles H = 7.62 moles = 1 = 1 so, the Empirical Formula = CH. As was presumed when we carried out this experiment, the vapor completely fills the flask - once the excess escapes (also flushing out the air). The volume of water that fills the flask equals the full volume of the flask and the volume occupied by the gas in the ideal gas law (V). V = volume of vapor = full volume of the flask = volume of water that fills it to the brim. V = Vwater filling flask = mass of water filling flask . dwater Recall that density is temperature -dependent. So, we must use the density of water at its temperature when it filled the flask. By the info in the problem, this temperature is 25ºC. Referring to the table of densities (Table 1), the density of liquid water at 25ºC is listed as: 0.997044 g/mL. 128.12 g V = Vwater filling flask = 0.997044 g/mL = 128.50 mL = 0.12850 L To determine the molecular formula, we need to find the molecular weight. The most useful form of the ideal gas law - considering the given information - is: MW = gRT ; T = 98 + 273 = 371 K and V = 0.1285 L. PV mass of condensed vapor = mass of gas in flask = 25.6803 g - 25.3478 g = 0.3325 g. MW = L atm. (0.3325 g) 0.0821 mole K (371 K) gRT = 78.0 g/mole. PV = 768.0 760 (0.12850 L) The EFW = Empirical Formula Weight for CH = 12.01 + 1.01 = 13.02 g/mole. N = # of empirical formula units = 78.0 g/mole MW = 13.02 g/mole = 5.99 = 6. EFW Thus, in this case, since N = 6, the molecular formula is 6 x Empirical Formula. Thus, the Molecular Formula = C6H6 . (The compound is benzene).