Lesson 5.3 ideal gas law
advertisement

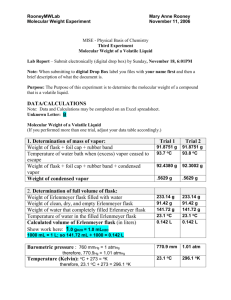
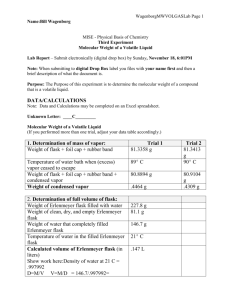
Lesson 5.3 The Ideal Gas Law Suggested Reading Zumdahl Chapter 5 Sections 5.3 Essential Questions What is the ideal gas law? Learning Objectives Solve problems using the ideal gas equation. Determine the density or molar mass of a gas from the ideal gas law. Introduction In the previous lesson, we discussed the empirical gas laws. Now we will show that these laws can be combined into one equation, called the ideal gas equation (or law). The Ideal Gas Law You learned in the previous lesson that the combined gas law combines Boyle's and Charles' laws into a single expression that describes basic properties of gases. The ideal gas law takes this one step further by incorporating Avogadro's law as well. The ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. The ideal gas law takes the form (see page 187 or your text book for the derivation if interested): PV = nRT P = Pressure of the confined gas in atmospheres V = Volume of the confined gas, in liters n = Number of moles of gas R = Gas Constant, usually 0.0821 L·atm/mol·K T = Temperature in Kelvin The ideal gas law includes all of the information contained in Boyle's, Charle's and Avogadro's laws. In fact, starting with the ideal gas las, you can derive any of the other gas laws. The limitations that apply to Boyle's, Charles' and Avogadro's laws also apply to the ideal gas law. That is, the ideal gas law is most accurate for low to moderate pressures and for temperatures that are not too low. When using the ideal gas law, we assume the ideal behavior of gases, however, no gas is "ideal". Despite this, the ideal gas law is a very useful even though is is only an approximation. The behavior or real gases will be discussed later. The Gas Constant The gas constant R takes a variety of forms, so you must be careful to choose the right one. Make sure volume and pressure units are in agreement with your data! The different variations mean that you can use a variety of units for volume and pressure, although temperature is always in Kelvin and amount is always expresses as moles. Calculations Using the Ideal Gas Law The type of problems to which Boyle's and Charles' laws are applied involve a changed in conditions (P, V, or T) of a gas. The ideal gas law allows us to solve another type of problem; given any three of the quantities, P, V, n, & T, we can calcuate the unknown quantity. Example: Using the Ideal Gas Law 5.0 g of neon is at 256 mm Hg and at a temperature of 35º C. What is the volume? Solution: Step 1: Write down your given information: P = 256 mmHg V=? m = 5.0 g R = 0.0820574 L·atm·mol-1K-1 T = 35º C Step 2: Convert as necessary: Pressure: Moles: Temperature: Step 3: Rearranging for the unknown and substituting gives (It is easier to rearrange the equation before plugging in. I strongly suggest you get in the habit of doing this!) Watch this You Tube Video: https://www.youtube.com/watch?v=yn7kmeD47Zc Gas Density The density of a substance is its mass divided by volume, and because the volume of a gas varies with temperature and pressure, the density of a gas also varies with temperature and pressure. The ideal gas equation can be used to calculate the density of a gas at any temperature and pressure. We do this by calculating the moles, and from this the mass in a liter of gas at the given temperature and pressure. The mass per liter of gas is its density. Example: Calculating Gas Density What is the density of oxygen, O2, in grams per liter at 25∘C and 0.850 atm? Solution: The density of a gas in g/L is equal to the mass of 1L (Recall from lesson 5.1 that this results from the property of expandability). Therefore, you can use the ideal gas law to calculate the moles, then mass, of 1L of O2to obtain its density. Step 1: Put the data into tabular form. P = 0.850 atm V = 1 L (exact value) T = 25 + 273 = 298K n=? Step 2: The unknown is n, so rearranging the ideal gas equation and then substituting gives: Step 3: Convert mol to grams Step 4: State the answer The density of oxygen at 25∘C and 0.850 atm is 1.11 g/L. Gas Density & Molecular Weight The density of a gas is directly proportional to its molecular weight. This relationship between the density and molecular weight of a gas suggests that you can use a measurement of gas density to determine molecular weight. In fact, gas-density (vapor-density) measurements provided one of the first methods of determining molecular weight. The method was worked out by Jean-Baptiste Dumas in 1826, and is commonly referred to as the Dumas method. It can be applied to any substance that can be vaporized without decomposing. You will carry out the Dumas method in this course. Lets look at an example. Example: Determination of Molecular Weight from Gas Density Determine the molecular weight of halothane, an inhalation anesthetic. The density of halothane vapor at 71∘C (344K) and 768 mmHg (1.01 atm) is 7.05 g/mL. Solution: To obtain the molecular weight (g/mol), you calculate the moles of vapor in a given volume. The molar mass equals the mass divided by the moles calculated. From the density, you see that one liter of vapor has a mass of 7.05 g. The moles of this volume are obtained from the ideal gas law. Therefore, the molar mass is The molecular weight is therefore 197 amu. To determine the molecular weight of a gas, all that you need to have is the mass of vapor of any given volume. An explicit determination of density is not necessary. You determine the moles of vapor from the volume using the ideal gas law, then determine the molar mass by dividing the mass by moles for this volume. This is what you will be doing in lab. Homework: Watch all gas law recording that go along with the power point notes slides. These are posted on the web site in chapter 5. Additionally, do practice exercises 10.9 – 10.13