CHEN 354- HW # 11- Due Monday, April 20, 2008
advertisement

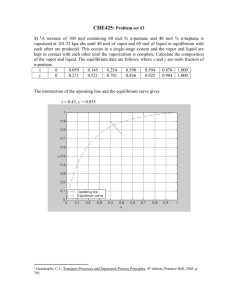
CHEN 354- HW # 11- Due Monday, April 21, 2008 Problem 1. The bubble point of a liquid mixture of n-butanol and water containing 4 mol% butanol is 92.7oC at 1.103 bar. At 92.7oC the vapor pressure of pure water is 0.784 bar and that of pure butanol is 0.427 bar. a. Assuming the activity coefficient of water in the 4 mol% butanol solution is about 1, what is the composition of the vapor in equilibrium with the 4mol%butanol-water mixture, and what is the activity coefficient of n-butanol? b. At 92.7oC, the maximum amount of n-butanol that can be dissolved in water is 4 mol%. When larger amounts of n-butanol are present, a second liquid phase containing 40 mol% n-butanol, appears. What are the activity coefficients for n-butanol and water in this second liquid phase? c. If the two coexisting liquids in part (b) are kept at 92.7oC, what will be the pressure when the first bubble of vapor is formed? Problem 2. Estimate the pressure and vapor-phase composition in equilibrium with a liquid whose overall composition is 50mol% fufural and 50 mol % isobutene at T = 37.8oC. Data: Piso(sat, at T=37.8oC) = 4.909 bar Pfurf(sat, at T=37.8oC) = 4.93 x 10-3 bar Isobutane and furfural are only partially miscible in the liquid phase. At 37.8oC, the two coexisting liquid phases contain 11.8 and 92.5 mol % isobutene, iso(xiso=0.925) = 1.019, and furf (xfurf = 0.882) =1.033. Problem 3. Ethyl alcohol and n-hexane are put into an evacuated, isothermal container. After equilibrium is established at 75oC, it is observed that two liquid phases and a vapor phase are in equilibrium. One of the liquid phases contains 9.02 mol % n-hexane. Activity coefficients for n-hexane-ethyl alcohol liquid mixtures can be represented by the equation RT ln i = 8.163 xj2 kJ/mol a. Compute the equilibrium composition of the coexisting liquid phase. b. Compute the equilibrium pressure and vapor phase mole fractions. Data: Vapor pressure equations (T in K, P in bar) ln PHsat = -3570.58/T +10.4575 ln PEOHsat = -4728.98/T + 13.4643 Problem 4. Ethanol and n-hexane form two liquid phases at many temperatures. Assuming the activity coefficients for n-hexane-ethyl alcohol mixtures can be represented by the equation RT ln i = 8.163 xj2 kJ/mol and that the vapor pressures of the pure components are as in Problem 2, compute the Tx-y diagram at pressures P= 0.1013 bar, 1.013 bar, and 10.13 bar. Problem 5. At moderate pressures, the virial equation of state can be used to estimate the solubility of a solid in a supercritical fluid. a. Compute the solubility of naphthalene in carbon dioxide at 50oC and 60 bar using the following data: At 50oC, the vapor pressure of naphatlene is 1.11x10-3 bar, its molar volume is 0.112 m3/kmol, and the virial coefficients are BCO2-CO2 = -0.103 m3/kmol BN-CO2 = -0.405 m3/kmol b. Repeat the computation in part (a) using the PR equation of state and compare the results.