CHEN 354-HW 2
advertisement

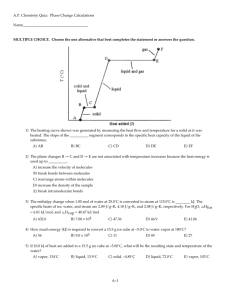
HW # 2—THERMO II—CHEN 354—Fall 08 Due Wednesday, September 10, 2008 Problem 1. For a separations process it is necessary to determine the VLE compositions of a mixture of ethyl bromide and n-heptane at 30oC. At this temperature the vapor pressure of pure ethyl bromide is 0.7569 bar, and the vapor pressure of pure n-heptane is 0.0773 bar. Calculate the bubble pressure and the composition of the vapor in equilibrium with a liquid containing 47.23 mol% ethyl bromide assuming ideal solution behavior. Compare the calculated pressure to the experimental value of 0.4537 bar. Problem 2. Consider a mixture of 50% mol n-pentane and 50% mol n-butane at 14 bar. (a) What is the dew point temperature? What is the composition of the first drop of liquid? (b) At what temperature is the vapor completely condensed if the pressure is maintained at 14 bar? What is the composition of the last bubble of vapor? Problem 3. A 50 mol% of propane (1) and n-butane(2) enters an isothermal flash drum at 37oC. If the flash drum is maintained at 0.6 MPa, what fraction of the feed exits as liquid? What are the compositions of the phases exiting the flash drum? Recommended additional problems: 10.17, 10.18, 10.19, 10.20. (These problems will not be graded and you don’t need to include the solution in your HW, just do them for practice).