Chemical Engineering Homework: Vapor-Liquid Equilibrium
advertisement

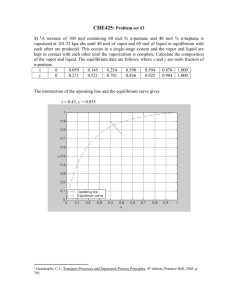
HW # 11-CHEN 354-Spring 10 Due Monday, April 12, 2010 Problem 1. Estimate the pressure and vapor-phase composition in equilibrium with a liquid whose overall composition is 50 mol % furfural and 50 mol% isobutene at 37.8oC. Data: Saturation pressures at 37.8oC : Pisosat =4.909 bar; Pfurfsat = 4.93x10-3 bar. Isobutane and furfural are only partially miscible in the liquid phase. At 37.8oC the two coexisting liquid phases contain 11.8 and 92.5 mol % isobutene respectively, iso(at xiso = 0.925) =1.019, and furf(at xfurf =0.882) =1.033. Problem 2. Ethyl alcohol and n-hexane are put into an evacuated, isothermal container. After equilibrium is established at 75oC, it is observed that two liquid phases and a vapor phase are in equilibrium. One of the liquid phases contains 9.02 mol% of n-hexane. Activity coefficients for nhexane-ethyl alcohol liquid mixtures can be represented by RT ln i = 8.163 xj2 kJ/mol a. Compute the equilibrium composition of the coexisting liquid phase. b. Compute the equilibrium pressure and vapor phase mole fractions Data: Vapor pressure equations (T in K, P in bar) ln Phexanesat = -3570.58/T + 10.4575 In PEOHsat = -4728.98/T + 13.4643 Problem 3. Ethanol and n-hexane form two liquid phases at many temperatures. Assuming that the activity coefficients of n-hexane/ethyl alcohol mixtures can be represented by the equation RT ln gi =8.163 xj2 kJ/mol and that the vapor pressures of the pure components are as in Problem 2, compute the T-x-y diagram at pressures P= 0.1013 bar, P= 1.013 bar, and 10.13 bar. Problem 4. At 25oC, a binary system is in a state of 3-phase VLLE. Analysis of the two equilibrium liquid phases ( and ) yields the following compositions: x2 =0.05; x1 = 0.01 Vapor pressures for the two pure components at 25oC are: P1sat = 0.7 bar; P2sat = 0.1 bar Stating your assumptions, determine reasonable estimates of: (a) the activity coefficients 1 and 2 (b) the equilibrium 3-phase pressure (c) the equilibrium vapor compositions y1 and y2 (d) the activity coefficients 2 and 1 (e) Sketch the P-x1 diagram at 25oC.