211-97 Acid-Base 102-3
advertisement

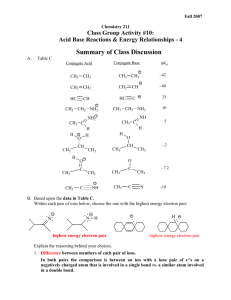
Chemistry 211 Fall 2013 Equilibrium Controlled Reactions: A. Acid Base Reactions and Electron Energies - 5 SUMMARY OF CLASS DISCUSSION Table C Conjugate Base Conjugate Acid pKa CH3 CH2 ~42 CH2 CH2 CH2 CH ~40 HC HC CH3 CH3 CH CH3 CH2 NH2 CH3 CH2 NH3 CH3 C 25 C NH NH2 CH3 C H H 10 5 Conjugate Acid H O H CH3 CH Conjugate Base O H CH3 CH 3 CH2 H O CH3 CH3 C CH pKa CH3 - 2 CH2 O CH3 CH3 N H CH3 CH2 C C C CH2 N CH3 - 7.2 -10 B. Based upon the data in Table C, Within each pair of structures below, choose the one with the highest energy electron pair: H N H H N highest energy electron pair highest energy electron pair Provide a warrant citing specific data in Table C. a. Difference between members of each pair of ions. In both pairs the comparison is between a structure with its HEE lone pair of e-'s on an atom that is involved in only -bonds vs. a similar atom involved in one or more -bonds. Acid-Base & Electron Energy Relationships- 5 2 b. Claims and Warrants from Table C data: The higher the bond order (triple bond > double bond > single bond) between the atom holding the proton to be lost and the next atom in the compound, the more acidic is the proton. This is true whether the acidic proton is on C, N or O (Table C: pKa's ~42 vs. ~40 vs. 25, 10 vs. 5 vs. -10 and -2 vs. -7.2 respectively) or is positive (Table C: pKa's 10 vs. 5 vs. -10 and -2 vs. -7.2) or neutral (pKa's ~42 vs. ~40 vs. 25). Least Acidic Most Acidic X Y X Y >> H - X Y X Y > H - X X Y Y H - Using the Relative Effect Assumption (As illustrated below.), we can conclude that energies of the conjugate bases increase in the order of those with HEE on atoms involved in a triple bond -> a double bond -> only single bonds. (pKa is determined by the energy of the HEE on the conjugate base.) C: C H H - C H G Base GO Acid Rxn. Coord. H C: Base GN G Acid Rxn. Coord. R-CH3 R-CH2Base GC G Acid Rxn. Coord. C. Suggest structural and/or theoretical arguments to explain the effects observed above. (See also CGWW p. 194 and Review of General chemistry and Mathematics #’s 29 & 30.) Backing: The structural differences in the molecules relate to the bond orders of the bonds between the atom holding the newly generated HEE lone pair e-‘s of the conjugate base and an adjacent heavy atom. To explain the energy differences among differently bonded species, we must have a theory that describes bonding in these molecules. So far we have been using a simple Lewis Dot Structure picture of molecular bonding. It has been useful for considering repulsive interactions among electron pairs on an atom or attractive interactions between electrons and nuclei, but in this case, descriptions of different electron environments on differently bonded atoms require a more sophisticated approach to describing molecular structure. One relatively simple bonding theory that was introduced in General Chemistry is Valence Bond Theory in which bonds are described as overlap of atomic orbitals and the differing geometries around heavy atoms are ascribed to differences in mixing of its atomic orbitals, hybridization, before bonds are formed between atoms. (See the CH 211 Review of General Chemistry and Mathematics #'s 29. and 30.) Acid-Base & Electron Energy Relationships- 5 3 Let's use the Valence Bond approach to describe the environments of the electrons in the molecules below. The hybridization of the orbital on the carbon atom, that is used to form the bond to the acidic proton of the conjugate acid and to which the newly formed lone pair electrons of the conjugate base is assigned, changes from sp to sp2 to sp3 as the number of sigma-bonds around the carbon atom increases from 2 to 3 to 4 respectively. The hybridization changes result in different proportions of s and p character in the hybrid orbital. So there is more s character in the hybrid orbital on the multiply bonded carbon atoms than in the singly bonded atoms. - e.g. H C C H H C H - C H C C H sp - hybridized sp orbital C H H sp2 - hybridized C H H C H sp2 orbital - H H H C H H H H C H sp3 - hybridized C H H sp3 orbital Electron distributions in s orbitals provide more effective interaction between their e-'s and the nucleus (effective nuclear charge) than do p orbitals in the same principle energy level. Consequently, as the s-character of a hybrid orbital increases, the effective nuclear charge felt by the e-'s in the orbital will increase. Thus, the energy of the e-'s will be lowered by increased attractive force. This effect is similar to the effect seen when the number of protons in the nucleus increases from C->N->O. Again, the decrease in energy of the e-'s is greater for the higher energy non-bonding electrons (lone pair e-'s in the conjugate base) than for the lower energy bonding electrons in the conjugate acid. Thus, increasing the s-character of the hybrid orbitals on the atom holding the proton lost, lowers the energy of the conjugate base form more than it does that of the lower energy conjugate acid form. So, increased s-character decreases G thus increasing the acidity of the acid. HC CH CH2 CH2 CH3 CH3 Base NOTE: Base Base G Gsp Acid Gsp2 G Acid Acid Rxn. Coord. sp pKa G Energy of nonbonding HEE electrons of the conjugate base Acidity of R-H Gsp3 G Rxn. Coord. sp2 Increase Increase Increases Increase Rxn. Coord. sp3 Magnitude of the Effect. There is a much larger difference between sp (50% s) & sp2 (33.3% s) atoms than between sp2 (33.3% s) & sp3 (25% s) atoms. The energy differences parallel the relative changes in % s-character of the orbitals. The overall pKa change from sp to sp3 for otherwise identical compounds is ~20 pK units. The change from sp to sp2 produces a change of ~15 pKa units, while the sp2 to sp3 yields a change of ~5 pKa units.