Solubility
advertisement

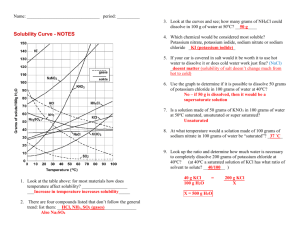
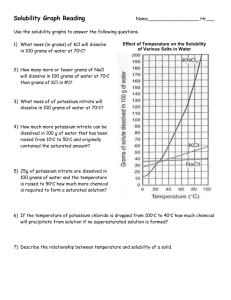
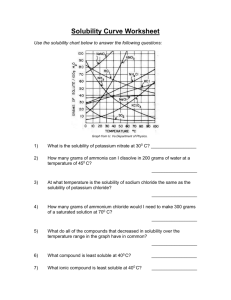
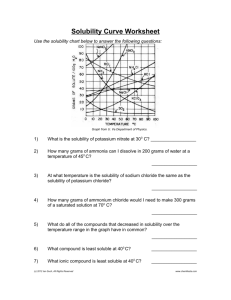
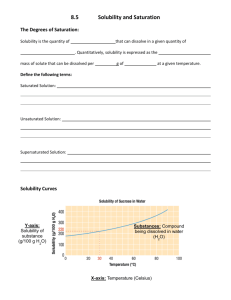
H Chemistry : Review Solubility Name __________________ period _____ 4/8/2013 Questions on Solubility : Using the Fig 10- 5. a) How much potassium chloride in grams can dissolve at most per 100 grams of water @ 78 degrees C? b) How much potassium chloride in grams can dissolve at most per 100 grams of water @ 78 degrees C? c) How many grams of sodium nitrate can dissolve at most per 100 grams of water @ 78 degrees C? d) e) If one added 20 grams of sodium chloride to 100 grams of water @ 20 degrees C, would the Solution be saturated, unsaturated or supersaturated? Explain f) At 10 degrees C, which salt sodium chlorate, sodium chloride or potassium chloride can be dissolved more in 100 grams of water? Given which one, how many grams can be dissolved at most at 10 degrees C? f)At what temperature C does the solubility of potassium nitrate equal the solubility of potassium chloride? g) At What temperature range( from what temp to what temp?) the solubility of sodium chloride exceed the solubility of potassium chlorate? h) If you were to add 60 grams of potassium nitrate @ 38 degrees C, how much would be dissolved at most if it was cooled to 20 degrees C? How much would precipitate out of solution?