UNCLE SLIM
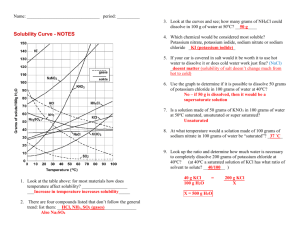
1)
Write the balanced equation for the reaction of Potassium Nitrate with Carbonic Acid to form
Potassium Carbonate and Nitric Acid:
2)
If you start with 50 grams of Potassium Nitrate and 100 grams of Carbonic Acid, how many
grams of Nitric Acid can be formed?
3)
What is the excess reactant in the reaction?
4)
How much of the excess reactant was used in the reaction?
PHAT JOE
1)
Write the balanced equation for the reaction of Potassium Carbonate and Nitric Acid to form
Potassium Nitrate with Carbonic Acid:
2)
If you start with 50 grams of Potassium Carbonate and 100 grams of Nitric Acid, how many
grams of Carbonic Acid can be formed?
3)
What is the excess reactant in the reaction?
4)
How much of the excess reactant was used in the reaction?
For chemistry help, visit www.chemfiesta.com!
© 2002 Cavalcade Publishing – All rights reserved