All labs are Microscale
advertisement

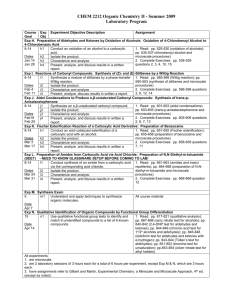
Organic Chemistry I Laboratory Program Summer 2008 Course Exp Experiment Objective Description Assignment Goal Obj Exp A. Introduction to the Organic Laboratory and Characterization Techniques 8-12 a1 Identify the safety features and physical layout of the 1. Scan: Ch 1-2. organic laboratory. 2. Read: instructor provided procedures; pp. 93-97 a2 Locate and operate instruments and equipment in the (recrystallization); pp. 106-107 laboratory. (benzoic acid); p. 108 a3 Recrystallize a pure sample of either benzoic acid or (naphthalene); pp. 113-117 naphthalene. (melting points); pp. 118-119 a4 Characterize benzoic acid, naphthalene, and a mixture (capillary-tube melting points); of these two by melting point analysis. pp. 175-180 (thin layer a5 Characterize common household products (Anacin, chromatography) Excedrin, Tylenol, aspirin, caffeine) using thin layer 3. Complete Exercises: pp. chromatography. 110-113 questions 8, 18, 31; pp. a6 Present, analyze, discuss results in a written report. 119-121 questions 3, 4, 14, 15; p. 184 questions 2, 3, 7 Exp B. Extraction of a Natural Product: Isolation of Trimyristin from Nutmeg 8-12 b1 Use extraction techniques to isolate a natural product. 1. Read: pp. 149-152 (theory of extraction); 167-168 b2 Characterize and analyze. (extraction of a natural product); b3 Present, analyze, discuss results in written a report. pp.168-170 (trimyristin and microscale procedures) 2. Complete Exercises: pp. 170-171 questions 2, 4, 5, 7, 8 Exp C. Acid-Base Extraction: Extract Components of a Mixture of an Acid (benzoic acid), Base (4nitroaniline), and Neutral (naphthalene) 8-12 c1 Use extraction techniques to isolate an acid, base, and 1. Read: pp. 152-157 (base and neutral compound. acid extractions); p. 162 (acidbase extraction microscale c2 Characterize and analyze. procedures) c3 Present, analyze, discuss results in a written report. 2. Complete: instructor provided pre-lab questions. Exp D. Bromination of Alkenes: Bromination of (E)-Stilbene 8-12, d1 Conduct an electrophilic addition reaction of alkenes. 1. Read: pp. 333-334 (alkenes 14 introduction); p. 372 d2 Isolate the product. (bromination of alkenes); pp. d3 Characterize and analyze. 372-374 (stilbene and d4 Present, analyze, discuss results in written a report. microscale procedures) 2. Complete Exercises: pp. 374-375 questions 1, 2, 9, 15 Exp E. Electrophilic Aromatic Substitution: Friedel-Crafts Alkylation of p-Xylene 8-12, e1 Conduct an electrophilic aromatic alkylation of an 1. Read: pp. 481-484 14 activated arene. (introduction, Friedel-Crafts alkylation), pp. 485-488 e2 Assess the relationship between carbocation (alkylation of p-xylene and rearrangement and arene nucleophilicity. microscale procedures) e3 Isolate the product. 2. Complete Exercises: pp. e4 Characterize and analyze. 488-493 questions 1, 3, 7, 10 e5 Present, analyze, discuss results in a written report. Exp F. Nucleophilic Substitution Reactions: SN1 vs SN2 8-12, f1 Review SN1, SN2, E1, E2 reactions and mechanisms. 1. Read: pp. 451-455 14 (nucleophilic aliphatic f2 Compare and contrast the effect of the leaving group CHEM 2211L Georgia Gwinnett College Page 1 of 2 2/15/2016 School of Science & Technology Organic Chemistry I Laboratory Program Summer 2008 and the substrate structure on nucleophilic substitution reactions of primary, secondary, and tertiary alkyl halides and aryl halides. Present, analyze, discuss results in a written report. substitution); Review McMurry pp. 371-389 (SN2 & SN1 reactions); pp. 392-400 (elimination reactions) f3 2. Complete instructor provided pre-lab questions. Exp G. Spectroscopy: Mass Spectroscopy (MS), Infrared Spectroscopy (IR), Ultra-Violet and Visible Spectroscopy (UV-Vis), and Nuclear Magnetic Resonance Spectroscopy (NMR) 8-12, g1 Learn to use the IR and UV-Vis instrumentation in the 1. Scan: pp. 233-238. 13 lab 2. Review: McMurray Chapters 11-12. g2 Understand and apply concepts and techniques from 3. Complete instructor provided MS, IR, UV-Vis, and NMR to analyze individual pre-lab exercises. spectra of each type. g3 Access spectra on web databases. g4 Integrate analysis of MS, IR, UV-Vis, and NMR to determine structure of unknown compounds g5 Present, analyze, discuss results in written report All experiments: 1. are microscale. 2. begin with a written quiz. 3. are 2 laboratory sessions of 3 hours each for a total of 6 hours per experiment. 4. require an individual student written report. 5. and assignments refer to Gilbert and Martin, Experimental Chemistry: a Miniscale and Microscale Approach, 4th ed. (except as noted). CHEM 2211L Georgia Gwinnett College Page 2 of 2 2/15/2016 School of Science & Technology