All labs are Microscale
advertisement

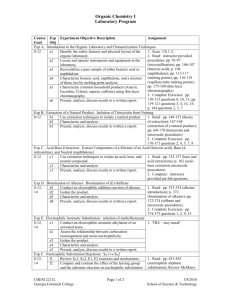
CHEM 2212 Organic Chemistry II - Summer 2009 Laboratory Program Course Exp Experiment Objective Description Assignment Goal Obj Exp H. Preparation of Aldehydes and Ketones by Oxidation of Alcohols: Oxidation of 4-Chlorobenzyl Alcohol to 4-Chlorobenzoic Acid 8-14 h1 Conduct an oxidation of an alcohol to a carboxylic 1. Read: pp. 525-530 (oxidation of alcohols); acid. pp. 535-537-chlorobenzyl alcohol and Dates microscale procedures) h2 Isolate the product. Jan 14 2. Complete Exercises: pp. 538-539 h3 Characterize and analyze. Jan 28 questions 2, 3, 4, 10, 15 h4 Present, analyze, and discuss results in a written report. Exp I. Reactions of Carbonyl Compounds: Synthesis of (Z)- and (E)-Stilbenes by a Wittig Reaction 8-14 i1 Synthesize a mixture of stilbenes by a phase-transfer 1. Read: pp. 585-589 (Wittig reaction); pp. Wittig reaction. 590-593 (synthesis of stilbenes and microscale Dates procedures) i2 Isolate the product. Feb 4 2. Complete Exercises: pp. 596-598 questions i3 Characterize and analyze. Feb 11 3, 6, 12, 14 i4 Present, analyze, discuss results in written a report. Exp J. Aldol Condensations to Produce α,β-unsaturated Carbonyl Compounds: Synthesis of trans-pAnisalacetophenone 8-14 j1 Synthesize an α,β-unsaturated carbonyl compound. 1. Read: pp. 601-603 (aldol condensations); pp. 603-605 (trans-p-anisalacetophenone and j2 Isolate the product. Dates microscale procedures) j3 Characterize and analyze. Feb18 2. Complete Exercises: pp. 605-606 questions j4 Present, analyze, and discuss results in written a Feb 25 2, 6, 7, 13 report. Exp K. Fischer Esterification Reaction of a Carboxylic Acid Derivative: Preparation of Benzocaine 8-14 k1 Conduct an acid-catalyzed esterification of a 1. Read: pp. 651-655 (Fischer esterification); carboxylic acid with an alcohol. pp. 655-658 (preparation of benzocaine and Dates microscale procedures) k2 Isolate the product. Mar 3 2. Complete Exercises: pp. 658-659 questions k3 Characterize and analyze. Mar 17 7, 11 k4 Present, analyze, and discuss results in written a report. Exp L. Preparation of Amides from Carboxylic Acid via Acid Chloride: Preparation of N,N-Diethyl-m-toluamide (DEET) - NEED TO KNOW GLASSWARE SETUP BEFORE COMING TO LAB! 8-14 l1 Conduct synthesis of an amide from a carboxylic acid 1. Read: pp. 661-663 (amides and insect via the corresponding acid chloride. repellents); pp. 664-668 (preparation of N,NDates diethyl-m-toluamide and microscale l2 Isolate the product. Mar 24 procedures) l3 Characterize and analyze. Mar 31 2. Complete Exercises: pp. 668-669 question l4 Present, analyze, and discuss results in a written 12 report. Exp M. Synthesis Exam 5 m1 Understand and apply techniques to synthesize All course material organic molecules. Date Apr 7 Exp N. Qualitative Identification of Organic Compounds by Functional Group Differentiation 15 n1 Use qualitative functional group tests to identify and 1. Read: pp. 817-821 (qualitative analysis); match 6 unidentified compounds to a list of 6 known pp. 867-868 (ceric nitrate test for alcohols); pp. Date compounds. 840-842 (2,4-DNP test for aldehydes and Apr 14 ketones); pp. 844-846 (chromic acid test for 1o/2o alcohols and aldehydes); pp. 846-848 (iodoform test for aldehydes and ketones with α-hydrogen); pp. 843-844 (Tollen’s test for aldehydes); pp. 851-852 (bromine test for unsaturation); pp.853-854 (silver nitrate test for alkyl halides) All experiments: 1. are microscale. 2. are 2 laboratory sessions of 3 hours each for a total of 6 hours per experiment, except Exp M & N, which are 3 hours each. 3. have assignments refer to Gilbert and Martin, Experimental Chemistry: a Miniscale and Microscale Approach, 4 th ed. (except as noted).