Review Sheet – Chemistry – Ch
advertisement

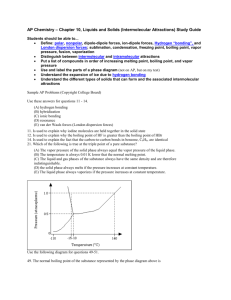
Review Sheet – Chemistry, Level 3 – Ch. 14: Liquids & Solids Name: _____ANSWERS_______________ Date:___________ Period:___ This test covers materials in the textbook in Chapters 14 (pp. 456-491). You are always responsible for the topics on the previous tests. Test Expectations The student should be able: Describe how the Kinetic-Molecular Theory of Matter predicts the properties of substances Analyze the forces and relative strengths of the forces between particles in a solid, liquid or gas and compare the substances in terms of basic properties such as melting point, boiling point, viscosity, surface tension, or vapor pressure Describe the type and properties of one of the four solids Draw or use phase diagrams or heating curves to describe phase changes 1. Define each of the following terms clearly and completely. (a) condensed state of matter – (s) amorphous solid (b) phase – (t) adhesion – (c) phase diagram – (u) cohesion – (d) heating curve – (v) covalent-network solid – (e) intramolecular force – (w) phase change – (f) intermolecular force – (x) vapor pressure – (g) metallic bond – (y) heat of fusion – (h) hydrogen bond – (z) heat of vaporization – (i) dipole-dipole force – (aa) sublimation – (j) dispersion force – (bb) deposition – (k) permanent dipole – (cc) vaporization – (l) temporary dipole – (dd) condensation – (m) induced dipole – (ee) phase change – (n) viscosity – (ff) freezing – (o) surface tension – (gg) melting – (p) water – (r) crystalline solid – (hh) Kinetic-Molecular Theory of Matter – 16. Water - Binary compound that occurs at room 1. Condensed state of matter – matter where the temperature as a clear colorless odorless tasteless particles are close together (solids or liquids) liquid. It is polar and forms hydrogen bonds. 2. Phase - A distinct state of matter in a system. 3. Phase Diagram - A graph, with axes representing in a regular manner, forming a three-dimensional temperature and pressure, showing the equilibrium array or lattice. conditions for a given substance to be a solid, liquid or gas. 4. Heating Curve – A plot of temperature verses time for a substance where energy is added at a constant rate. 5. 6. 7. not arranged in a definite crystal structure. 19. Adhesion - The tendency of certain dissimilar molecules to cling together due to attractive forces. 20. Cohesion - The intermolecular attraction between like-molecules within a body or substance that acts covalent, and metallic to unite them. Intermolecular Forces – Attractive forces between 21. Covalent-network Solid – A substance which consists separate covalent molecules. of an array of atoms held together by an array of Metallic Bond - A chemical bond in which electrons covalent bonds. 22. Phase Change - A change from one state (solid or conduction occurs. liquid or gas) to another without a change in Hydrogen Bonds - A hydrogen bond results from a chemical composition. dipole-dipole force between a small electronegative atom and a hydrogen 9. 18. Amorphous Solid - A solid in which atoms or ions are Intramolecular Forces – Chemical bonds: ionic, are shared over many nuclei and electronic 8. 17. Crystalline Solid - A solid whose atoms are arranged Dipole-dipole Forces – Attractive forces between the 23. Vapor Pressure - The pressure that a vapor exerts, or the partial pressure if it is mixed with other gases. 24. Heat of Fusion – The amount of energy required to positive end of one polar molecule and the negative transform a substance from a liquid state to a solid end of another polar molecule. state. 10. Dispersion Force - Intermolecular forces arising from an instantaneous dipole in one molecule inducing a dipole in another molecule. 11. Permanent Dipole – A polar molecule. 12. Temporary Dipole – A molecule where a temporary imbalance in the electron cloud causes a momentary imbalance of charge. 13. Induced Dipole - Formed by the repulsion of electrons in an σ-bond by the high electron density in a neighboring molecule. 14. Viscosity - The measurement of the resistance of a liquid to flow. 15. Surface Tension - The result of attraction between molecules of a liquid which causes the surface of the liquid to act as a thin elastic film under tension. 25. Heat of Vaporization – The amount of heat energy required to convert water from a liquid to a gas. 26. Sublimation – A change directly from the solid to the gaseous state without becoming liquid 27. Deposition – Deposition is a process in which gas transforms into solid. 28. Vaporization –A phase transition from the liquid phase to gas phase. 29. Condensation – The process of changing from a gaseous to a liquid or solid state. 30. Phase Change –The process by which a substance transforms from solid to liquid or liquid to gas. (repeat) 31. Freezing –The change in state of a substance from 33. Kinetic-Molecular Theory of Matter – The state of liquid to solid by cooling to a critically low matter at a given temperature depends on the temperature. strength of attraction between its particles. 32. Melting – A process that results in the phase change of a substance from a solid to a liquid. 2. Explain the following in one to three sentences. (a) Water’s boiling point is lower in Denver than in Southington. The atmospheric pressure in Denver is lower and since boiling occurs when the vapor pressure of a liquid equals its atmospheric pressure, then water bowls at a lower temperature in Southington. (b) Graphite is a conductor, but diamond is an insulator. Graphite is a covalent-network solid that occurs in sheets. These sheets provide an opportunity for easy movement of electrons between the carbon atoms. Diamond occurs in a three-dimensional matrix and electrons don’t have an easy path to flow. (c) C5H12 has a higher boiling point than C3H8. Both of these substances are completely nonpolar. Nonpolar substances only have dispersion forces. Since dispersion forces are stronger for higher molar mass substances, then C5H12 has stronger forces and boils at a higher point. 3. For each of the solids listed, give I. the type of solid (ionic, molecular, metallic, or network) II. the particles that occupy the corners of the crystal lattice III. the bond or force that holds the solid together. (a) NH3 I. molecular II. Molecules III. Intermolecular forces (h-bonds) (b) Ag I. metallic II. Atoms III. Metallic bonds (c) Ge I. covalent-network II. Atoms III. Covalent bonds (d) Sn I. metallic II. Atoms III. Metallic bonds (e) KBr I. ionic II. Ions III. Ionic bonds 4. Which type are each of the solids described below? (a) crystalline solid with slight odor that melts at 78◦C molecular (b) solid that conducts in the solid phase metallic (c) solid with a very high melting point that does not conduct covalent-network (d) solid that does not conduct but dissolves in water to make a conducting solution ionic 5. For the phase diagram at the right, name the phase or phases that exist at each of the lettered points. (A) gas (B) gas/liquid (C) triple point (gas/liquid/solid) (D) liquid (E) solid/liquid 6. Referring to the phase diagram in # 5, what change or changes would occur if (a) one started at point A and raised the pressure? The gas condenses to a liquid. (b) one started at point B and raised the temperature? The liquid vaporizes to a gas. 7. Referring to the phase diagram in # 4, which is more dense, the solid or the liquid? How do you know? The liquid is less dense. You can tell because the solid/liquid line slopes towards the liquid. 8. Explain the following in one to three sentences. (a) Which liquid, Hg or H2O, has a greater surface tension? Hg has stronger forces (metallic bonds), so it has a greater surface tension. (b) Which liquid, H2O or C5H12, has greater viscosity? Water has a greater viscosity because it has hydrogen bonds versus dispersion forces. (c) Which liquid, HCl or HF, has a higher boiling point? HF has a higher boiling point because it has hydrogen bonds versus regular dipole-dipole forces in HCl. (d) Which liquid, C5H12 or C8H18, has a higher vapor pressure? C5H12 has a higher vapor pressure because it has a lower molar mass and therefore weaker dispersion forces. 9. Draw a phase diagram for the substance with the following properties. I. normal melting point: -17◦C II. normal boiling point: 57◦C III. triple point: -22◦C and 200 mmHg IV. critical point: 220◦C and 1400 mmHg See Mr. Niro if you have questions on # 9. 10. Draw a heating curve for the substance in # 9 at 1 atm of pressure. See Mr. Niro if you have questions on # 10.