HPLC, LC-MS, & IC
advertisement

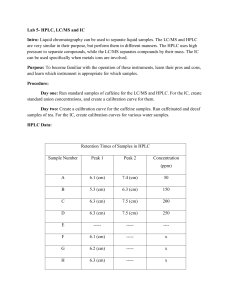
HPLC, LC-MS, & IC Introduction HPLC is High Pressure Liquid Chromatography. It is generally used for the separation of compound mixtures, as well as for identification, quantity, and purity measurements. A column separation technique is used, in which the analyte is separated by mobile and stationary phases under extreme pressure. A detector gives a chromatograph with retention times. Procedure Day 1: Investigate the operation of the HPLC and LC-MS by running standard samples of caffeine and note retention times. Day 2: Create calibration curve for caffeine. Run samples of caffeinated and decaf tea. Preparation: 1) Ensure that there is enough degassed methanol and water, the lines coming from the degassed species have no air bubbles, the computer is still on, and the injector knob is towards the left on load 2) Turn on the detector 3) 1g of caffeine goes into 1000mL water to make 1000ppm solution Software: 1) Click the HPLC icon on the desktop 2) Select Shimadzu 3) In the program window, click on the Methods icon at the top 4) In the Methods window, select the method: TSW.met 5) Click on the Parameters icon at the top a) Flow rate A: .500 mL/min b) Flow rate B: .500 mL/min c) Flow rate C: 1.000 mL/min d) Max pressure: 6000psi e) Min pressure: 1 psi f) Wavelength: 256nm 6) Click Download – Ok Running Samples: 1) Prepare sample for injection by drawing it into a syringe 2) Insert into injection port but do not inject or turn the knob yet 3) Click on the Run Single icon at the top of program window 4) In the new window, assign file name with initials and click start 5) When the bottom of the window says “waiting for trigger,” the sample can be injected 6) Inject until about 3-5 drops of sample fall from waste hose, switch knob to inject, and leave syringe in 7) Program will run for about 5 minutes Results: 1) Click red stop sign icon at top 2) Click analyze icon at top and label peaks on chromatogram 3) Click report icon at top and Area % in dropdown menu Shutdown: 1) Click method icon and select: LOWFLOW.met 2) Click on parameters icon and ensure these values are assigned: a. Flow rate A: .01 mL/min b. Flow rate B: .01 mL/min c. Flow rate C: 1.000 mL/min 3) Click download, then exit program 4) Ensure screens for A & B are at .01mL/min 5) Turn off detector Results: Retention Times of Samples in HPLC Sample Number Peak 1 Peak 2 Concentration (ppm) A 6.1 (cm) 7.4 (cm) 50 B 5.3 (cm) 6.3 (cm) 150 C 6.3 (cm) 7.5 (cm) 200 D 6.3 (cm) 7.5 (cm) 250 E ----- ----- ---- F 6.1 (cm) ----- x G 6.2 (cm) ----- x H 6.3 (cm) ----- x We did not get an area output so we could not make a calibration curve. Because of this, we had to base our conclusions on retention times. All of the unknowns only had one peak and the standards had two peaks. The standard peaks were at about 6.2cm and 7.4cm. The second peak was the characteristic caffeine peak. Conclusion Our unknown samples did not have caffeine peaks, most likely because our samples were made ahead of time and stored so the caffeine may have degraded. Because of this, we were not able to determine the amount of caffeine in the unknowns. If we were to do the experiment again, we would make fresh samples instead of using the same ones throughout the experiment. Introduction LC-MS stands for Liquid Chromatography-Mass Spectrometry. It uses a similar column separation technique to the HPLC, but with mass analysis. After separation, the solvent is removed and the sample is ionized. A mass detector scans the molecules by mass and produces a spectrum that identifies compounds within samples. Procedure Sample Preparation: Small vials are to be used that can be filled to a total volume of 1.5mL. These vials are to be capped and placed into the numbered tray. 1) Make caffeine solution of 1000ppm 2) Make dilutions using M1V1=M2V2 equation: concentrations are 50ppm, 100ppm, 150ppm, 200ppm, 250ppm, 300ppm 3) Unknown samples made from 1mL tea in 100mL water Sample Preparations of Standards and Unknown Vial Number # (mL) Of Stock Dilution Flask Desired Retention Solution (mL) Concen. (ppm) Times (min) A 5 (mL) 100 (mL) 50 ppm 1.8480 (min) B 10 (mL) 100 (mL) 100 ppm 1.8457 (min) C 15(mL) 100 (mL) 150 ppm 1.8575 (min) D 20(mL) 100 (mL) 200 ppm 1.8602 (min) E 25(mL) 100 (mL) 250 ppm 1.8606 (min) F 30 (mL) 100 (mL) 300 ppm 1.8540 (min) G 1 (mL) 100 (mL) (x) 1.7056 (min) H 1 (mL) 100 (mL) (x) 1.7579 (min) L 1 (mL) 100 (mL) (x) 1.6862 (min) Preparation: 1) Ensure that all sections of instrument are powered on 2) Turn on nitrogen in gas room to about 40psi 3) Open nitrogen valve at bench top 4) Make sure flasks are full Software: 1) Click analyst software icon on desktop 2) In vertical menu, click on hardware configuration – LCMS – activate profile (green) 3) Click build acquisition batch and set file name 4) Select method “cafeine10” 5) Click add set – add sample and select number of samples 6) Under vial position, enter numbers where vials are 7) Click on submit tab – submit – view and select “sample queue” – ready – start sample 8) Check LC pump to ensure it has pressure Results: 1) In left panel, click on open data file and select set name 2) After graph appears, right click on it and click on list data 3) Click on peak list tab Shutdown: 1) In left vertical menu, click on hardware configuration 2) Click on LC-MS 3) Click deactivate profile to shut down instrument 4) Shut off nitrogen gas valve at desk and in gas room Calculations: 1) M1V1=M2V2 (1000ppm)(x) = (50ppm)(10mL) X= 5mL 2) Y=mx+b Sample G 528110 = 117476(x) – 505400 X = 8.7976ppm Sample H (480140) = (117476)(x) – 505400 X = 8.3989ppm Sample I (515490) = (117476)(x) – 505400 X = 8.6902ppm Results: LC-MS Results For Caffeine Standards (A-F) and Unknown Tea Samples (G-H) Vial Number # Concentration (ppm) Area (Counts) A 50 (ppm) 5495900 B 100 (ppm) 2853700 C 150 (ppm) 20375000 D 200 (ppm) 23934000 E 250 (ppm) 31307000 F 300 (ppm) 33924000 G unknown 528110 H unknown 480140 I unknown 515490 A standard curve of Area vs. Concentration was made in order to analyze our results. The standard curve could also be used to find concentration in unknown samples using the equation y=mx+b Conclusion: The concentrations of the caffeine samples were determined to be about 8.4ppm, 8.8ppm, and 8.7ppm. Sample H was decaffeinated and not supposed to have a caffeine peak so there could have been some contamination. The retention times of the samples did not exactly match the standards, but they were fairly close. The peaks, however, were consistently found in the 1.7-1.8 area. The concentration of our unknowns may have been too diluted, which could have affected retention time. Introduction Ion chromatography is a form of liquid chromatography that is used to analyze ion levels in aqueous samples. It contains a column that separates anions. Procedure Day 1: Investigate the operation of the IC by creating standard anion concentrations. Create a calibration curve using an anion mixture and run the standards. Day 2: Create a new calibration curve and run standards followed by a variety of water samples *Water samples were obtained from the lab, men’s bathroom, and women’s bathroom Experimental 1. Check Helium tank in gas room. Pressure should be at 80 psi or above. 2. A pressure gauge on the instrument should be receiving helium and reading 9 psi. Turn knob near the gauge to correct if needed. 3. Regenerant and Eluent bottles on the instrument should be full. If less than half full they need to be refilled. a. Gas must be turned off to refill either bottle. b. Regenerant is made by diluting 1.7mL of sulfuric acid to 2 L volumetric flask in distilled water. c. Eluent is prepared by diluting 10mL of the eluent (in a bottle near the 7 ion standard) to 1 L volumetric flask in distilled water. 4. Open Chromeleon program from desktop. 5. From the open program, Click: File→ Open. a. In the new menu change the file type to control panel. Click the MyPannels folder and click to open the ICS-90 system. 6. Turn the pump off 7. Turn the small black knob on the pump counter clockwise for 30 sec to purge the system 8. Turn the pump back on in the program window and continue to purge for 30 sec 9. Turn knob on pump clockwise 10. Stop and let the system equilibrate itself for 20 to 30 minutes 11. After the allotted time, check the hoses below. If eluent collects 1 mL over a full minute, proceed with procedures. 12. A new sequence is started by clicking, File→ New→ Sequence. 13. Just click next in the box that appears. 14. Injection parameters: a. Select number of vials to be used b. State the number of injections of each vial c. Start position is 2 d. Injection volume is 10μL e. Click, Apply→ Next f. Name your sequence with initials and click done. 15. Click Batch Menu→ Edit→ add. There should be a list of sequences, find yours and click start. a. A window will appear telling you to inject the sample, do so now till a small amount drips out into a waste beaker 16. Click OK on the window. Each run will take 16 minutes 17. To open the sequence, click: File→ open→ select sequence→ your sequence name (with initials) 18. Double click on the 7 anion standard in a window that has the names of your injections listed. 19. In this new window click on the peak analysis tab a. Double click on the first positive peak b. After a box appears, pull the blue sheet from the drawer below and name the peaks from the sheet. 20. Ensure the instrument has stopped running by opening the batch menu and clicking stop. 21. Leave Chromeleon program running 22. Check eluent and regenerant levels, close the program by clicking the close button at the top right of the program window. Results Seven Ion Standard Data Peak 1 Peak Name Retention Time (min) 1 Fluoride 3.01 2 Chloride 3.60 3 Nitrite 3.93 4 Bromide 4.45 5 Nitrate 4.75 6 Phosphate 5.24 7 Sulfate 5.77 Retention Times (min) of Water Sample Data Sample Name Peak 1 Peak 2 Peak 3 Peak 4 Peak 5 Peak 6 Lab Water 2.30 (min) 3.59 (min) 4.76 (min) 5.75 (min) -- -- Men’s BR 2.28 (min) 3.14 (min) 3.56 (min) 4.72 (min) 5.70 (min) 11.99 Water The lab water was shown to contain chloride, sulfate, and nitrate. The retention times of chloride and sulfate appeared to match up with the seven ion standard samples. The results of the bathroom sample were shown to contain fluoride, chloride, nitrate, and sulfate. (min) Conclusion Although the results seemed to show what each of the samples contained, we cannot be sure that these were accurate because there were additional unknown peaks present in the spectra. These unknown peaks did not match up with any of the known retention times.