Sample Laboratory Report

APPENDIX III
SAMPLE LAB REPORT
Experiment 1
High Performance Liquid Chromatography
Alfred E. Neuman
Chemistry 325
January 5, 2004
INTRODUCTION
In this experiment, high performance liquid chromatography (HPLC) was used to separate a mixture of three similar alkaloids: caffeine, theobromine, and theophylline. The retention times of the three compounds were measured, and a calibration model for caffeine was constructed on the basis of the chromatographic peak heights produced by a series of six caffeine standard solutions. The derived calibration model was then employed in the prediction of caffeine content in a commercial soft drink.
PROCEDURE
Instrumentation and Equipment. A modular HPLC setup was used in this experiment, consisting of (1) a Milton-Roy Model 1100 pump, (2) a 20 uL sample injector, (3) an Altex C-8 reverse phase HPLC column, (4) an ultraviolet detector operating at 254 nm, and (5) an output chart recorder. In solution preparation, a Mettler AE-160 analytical balance was used to weigh solid reagents. Class A volumetric glassware was used throughout the experiment. separate 1 L solutions of caffeine, theophylline, and theobromine were prepared in deionized water. The weights of solute used were 0.1011 g, 0.1022 g, and 0.0998 for caffeine, theophylline, and theobromine, respectively.
In constructing the calibration model for caffeine, six standard solutions were prepared by dilution of a caffeine stock solution. The stock solution was prepared by dissolving 0.5000 g of caffeine in enough deionized water to make 1 L of solution. Employing 5, 10, 15, and 20 mL volumetric pipettes, aliquots of 5, 10, 15, 20, 30, and 40 mL were removed from the stock solution and diluted to 100 mL with deionized water. The unknown for the caffeine determination was a sample of "Pepsi", a commercial soft drink.
Data Collection and Analysis. A mixture of acetonitrile (20% v/v) in deionized water was used as the mobile phase. This solution was degassed prior to being used. A flow rate of 1.0 mL/min was employed during the equilibration and during subsequent injections of the samples.
For the study of the retention times of the three alkaloids, three injections of each solution were made. In constructing the caffeine calibration model, three injections were made of each of the six standard solutions. Three injections of the "Pepsi" unknown were then made.
No dilution of the unknown was made before injection. The recorded chromatograms were analyzed by measuring peak heights with a centimeter ruler.
EXPERIMENTAL DATA
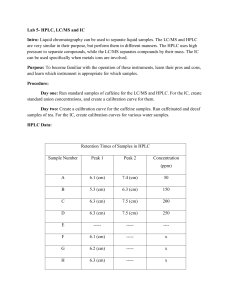
Retention Time Study
Retention Time (sec, ± 0.1 sec)
Compound Trial 1 Trial 2 Trial 3
Caffeine 180.1 181.3 180.5
Theobromine 154.6 155.3 154.9
Theophylline 244.7 245.8 245.2
III-2
Caffeine Calibration Study
Aliquot Volume Peak Height (cm, ± 0.05 cm)
(mL) Trial 1 Trial 2 Trial 3
5.0 2.10 1.25 1.50
10.0 3.82 3.20 3.80
15.0 5.02 5.09 5.60
20.0 8.40 8.35 7.70
30.0 11.90 10.25 11.05
40.0 14.55 15.34 13.80
Unknown 6.23 6.58 6.61
CALCULATIONS AND RESULTS
Retention Time Study
The retention times of caffeine, theobromine, and theophylline were analyzed to compute the mean retention time, standard deviation of retention time, and % relative standard deviation (%RSD). Means and standard deviations were computed by use of functions resident in Microsoft Excel.
Table I
Results of Retention Time Study
Compound Mean Retention Time Standard Deviation % RSD
(sec) (sec)
Caffeine 180.6 0.6 0.39
Theobromine 154.9 0.4 0.23
Theophylline 245.2 0.6 0.22
Caffeine Calibration Study
Calculation of Exact Concentrations of Standards
C stock
= (0.5000 g caffeine/1.0 L) x (1000 mg/g) = 500.0 mg/L = 500.0 ppm
For a given standard,
C std
= (A)(C stock
)/100 mL where A is an aliquot volume in mL.
Example: (Standard #1) C std
= (5 mL)(500.0 ppm)/(100 mL) = 25.0 ppm
III-3
Table II
Exact Concentrations of Standards
Standard # Concentration (ppm)
1 25.0
2 50.0
3 75.0
4 100.0
5 150.0
6 200.0
Calculation of Mean Peak Heights and Confidence Limits
The means and standard deviations of the three replicate peak heights for each concentration standard were computed by use of the built-in functions of Microsoft Excel. The 95% confidence limits were computed as
Upper Limit = Mean + t *s / n
½
Lower Limit = Mean - t *s / n
½ where Mean is the computed mean peak height for a given standard, s is the corresponding computed standard deviation, n = 3 replicates, and t = 4.303 for n - 1 = 2 degrees of freedom
(Microsoft Excel function “tinv”)
Table III
Mean Peak Heights, Standard Deviations, and Confidence Limits (CL)
Concentration Mean Peak Height S. Deviation Upper 95% CL Lower 95% CL
(ppm) (cm) (cm) (cm) (cm)
25.0 1.62 0.44 2.71 0.53
50.0 3.61 0.35 4.48 2.74
75.0 5.24 0.32 6.04 4.45
100.0 8.15 0.39 9.12 7.18
150.0 11.07 0.83 13.13 9.01
200.0 14.56 0.77 16.47 12.65
Calculation of Calibration Model
A least-squares calculation was used to compute the slope and intercept of the best calibration model for the above data. The resident least-squares functions of Microsoft Excel were used for this calculation.
Peak Height = -0.0486 + (0.074220)(Concentration)
To evaluate the quality of the calibration model, the correlation coefficient and the standard error of estimate were computed. The built-in functions of Microsoft Excel were used for these calculations.
III-4
r
2
= 0.9948 s = 1.14 cm
Plot of Calibration Model
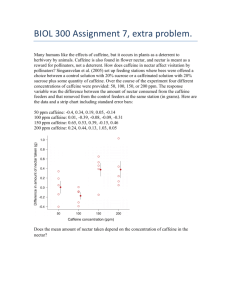
A plot of the calibration model is included as Figure 1. This plot was generated with the commercial software package, Axum (Version 3.0), operating on a Dell 466/L computer. The data values from Table II are plotted along with the computed regression line.
Figure 1 : Mean peak height (cm) vs. caffeine concentration (ppm). Error bars are plotted as the 95% confidence intervals based on three replicate peak height measurements at each concentration. The solid line identifies the computed regression line.
Computation of Unknown Caffeine Concentration
Each peak height of the unknown was used in conjunction with the computed calibration model to obtain a concentration estimate. An example is provided below for trial 1:
C unk,1
= (6.23 + 0.0486)/0.07422 = 84.6 ppm
The resulting estimates for the unknown were 84.6, 89.3, and 89.7 ppm. These estimates were used with the built-in functions of Excel to compute an average, standard deviation, and 95% confidence interval for the unknown concentration:
III-5
Average: 87.9 ppm
Standard deviation: 2.8 ppm
95% confidence interval: 7.1 ppm
Applying the confidence interval, the unknown concentration in the “Pepsi” sample was estimated with 95% confidence to lie in the range of 80.8 to 95.0 ppm.
Questions
1. Two explanations for constituents not showing up in the chromatograms are (1) their possible co-elution with other peaks in the chromatogram or (2) their being permanently retained on the column. It is also possible that constituents may not absorb light at 254 nm, the wavelength used by the detector employed here.
2. Enhanced qualitative structural information would best be obtained by use of an alternate detector. Mass spectrometric detection would perhaps give the most structural information.
DISCUSSION
The calibration model produced an excellent fit to the experimental data, as evidenced by the high value of the correlation coefficient. While no formal test of accuracy was performed, the successful calibration model lends confidence to the determined caffeine value. A trend in the calibration model is an increase in the peak height errors (i.e., larger confidence intervals) with increasing concentration. This could indicate imprecision in the injection volume. The use of an internal standard might remedy this problem.
The retention time study indicated clearly that the three compounds could be separated using the C8-column. This study also revealed that the retention times were quite reproducible
(relative standard deviations < 1%). No anomalies were encountered during the experimental procedure.
III-6