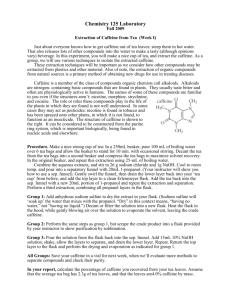
Figure I : Diagram of a Typical HPLC
advertisement

EXPERIMENT VIII HIGH PERFORMANCE LIQUID CHROMATOGRAPHY SEPARATION AND ANALYSIS OF MIXTURES These files are in Adobe Acrobat format, if you are using Netscape Navigator or Internet Explorer and have Adobe Acrobat Reader installed (If you do not; Acrobat Reader can be downloaded for free from Adobe) these files should open directly in your browser. INTRODUCTION Increasingly, the determination of low concentrations of active ingredients (either desired or undesired) in complex mixtures, sold for human consumption, has become more necessary. Federal regulations have imposed strict limits on the type and concentrations of a host of substances sold as foods or drugs. Such requirements demand analytical techniques that are fast and reliable and combine the separation (to alleviate interferences) and analysis steps in a single operation. Chromatography is the most widely used technique for the analysis of non-inorganic mixtures. Gas chromatography (where the sample must be volatilized) and liquid chromatography (where the sample can be determined in the liquid state) are the most common methods in general use. High Performance Liquid Chromatography (HPLC) is the method of choice whenever the sample cannot easily be converted to the gas phase. In this laboratory, you will use the technique of HPLC to determine either the concentration of caffeine in a soft drink, coffee or tea, or caffeine in one of a variety of analgesic (pain relief) formulations. THEORY Figure I depicts the main components of a modern HPLC system and their interrelationships. In HPLC, a solution containing the compound(s) of interest is injected into a loop which has been calibrated to contain a specified volume (a 20 L loop injector is a commonly used size). The valve switch is then rotated, allowing a sample stream of mobile phase (the eluent) to sweep the sample from the loop onto a column, packed with a suitable stationary phase, where the separation occurs. The eluent is delivered from a pump at a constant rate, (on the order of 1 mL/min) at a pressure sufficiently high to overcome the backpressure of the column. Pressures of 1000-2000 psi are commonly necessary. An upper limit of 4000 psi is normally set on the instrument. Recall that the separation efficiency is inversely proportional to the particle size of the column packing material. High pressures are required to force a liquid through a tightly-packed column filled with small particle material, and the availability of high pressure solvent delivery systems is directly responsible for the "high performance". Assuming that a suitable column has been chosen for the separation of interest, all components should pass through the column and "elute" at different times (differential migration). This time differential is due to the differences in the distribution (partitioning) of the various components between the mobile phase (eluent) and stationary phase (column packing), which arise from the physical/chemical differences among the components of the mixture. Thus, each component will pass through the detector and be identified separately. The time for a substance to pass through the column, termed the retention time, is therefore, related to the identity of the compound and is the basis for qualitative analysis. Quantitative information is obtained from the area or height of the peak produced by the detector. Figure I : Diagram of a Typical HPLC Several different approaches to HPLC detection exist. Perhaps most common is a detector based on the absorbance of ultraviolet or visible light, a UV/Vis absorbance detector. These detectors are, in reality, miniature UV/Vis spectrophotometers, equipped with a flowthrough cell, allowing continuous monitoring of the eluent. The wavelength selected corresponds to the region of the electromagnetic spectrum where the compound(s) of interest and/or their associated chromophores absorb light. In the linear dynamic range of the calibration curve the absorbance is proportional to the concentration of the compound of interest. The data is recorded and presented using a chromatographic integrator. The absorbance measured by the detector produces a peak with a characteristic retention time; each peak has an area which is listed as well. Measurement of a series of standards, along with the unknown, allows the use of the "standard series" method, which produce the calibration curve to determine the concentration of the unknown compound. PART I DETERMINATION OF CAFFEINE IN AN ANALGESIC TABLET PROCEDURE Preparation of Eluent If the eluent solution is not already prepared, make approximately 250 mL of an aqueous 1% (v/v) acetic acid solution using HPLC-grade water. Add approximately 160 mL of HPLC-grade methanol to this solution; the resultant 60/40 (v/v) solution can be used as the eluent and transferred to the appropriate solvent reservoir. Sparge the mobile phase liquid with helium prior to use. Preparation of Standard Solutions Weigh approximately, but accurately 250 mg of Caffeine, transfer quantitatively to a 250-mL volumetric flask and dilute to the mark with HPLC grade methanol to make an approximate but accurately known 1000 ppm. solution. Dilute 50 mL of this solution to 500 mL. The concentration of this solution should be approximately 100 ppm and should be known to three significant figures. This is the stock solution. From the stock solution prepare by successive dilution six solutions, each in a 100-mL volumetric flask, of concentrations 10, 20, 40, 50, 60, and 80 ppm respectively, using HPLC grade methanol as the dilutant. Your concentrations do not have to be exactly these values but they must be accurately known. Preparation of Sample Accurately weigh (+0.1 mg) one tablet of your over-the-counter analgesic. Dissolve this tablet in HPLC-grade methanol in a 100-mL volumetric flask and dilute to the mark with methanol. This is the unknown solution. Preparation of the Calibration Curve Following the instructors' guidelines, fill the sample loop with the most concentrated standard solution, inject and record the chromatogram, taking special note of the retention time and peak area of the caffeine peak. Repeat this procedure with each of the other standard solutions as well as the blank (pure methanol). Determination of the Caffeine in the Analgesic Preparation Obtain a chromatogram of the unknown solution using the same procedure as was used for the preparation of the calibration curve. CALCULATION OF RESULTS You will submit the results of this experiment in the form of a laboratory report (see p.p. 1-3 of these laboratory instructions). The data will fit a graph of standardized peak area vs. concentration. If appropriate, you should perform a linear regression analysis to determine the sensitivity of the method (slope) and the concentration. Be certain to correct for dilution of the original sample and report the results as mg caffeine/tablet. PART II DETERMINATION OF CAFFEINE IN A SOFT DRINK, COFFEE OR TEA PROCEDURE The eluent, standard solutions instrument settings and approach to preparing a calibration curve are identical to that given in PART I of this experiment. The unknown will consist of two different brands or types of soft drink, coffee or tea. Ideally, one would be "regular" and the other "caffeine-free". The soft drink coffee and tea may be analyzed directly (no dilution). It is, however, recommended that for the soft drink it be allowed to stand in an open container for at least ½ hour to lose its carbonation (CO2). CALCULATION OF RESULTS You will submit the results of this experiment in the form of a laboratory report (see p.p. 1-3 of these laboratory instructions). The data will fit a graph of peak area vs. concentration. If appropriate, you should perform a linear regression analysis to determine the sensitivity of the method (slope) and the concentration. Report the results of the analysis as mg caffeine/12-oz. serving of [Brand Name].