CHAPTER 8 – ACIDS & BASES
advertisement

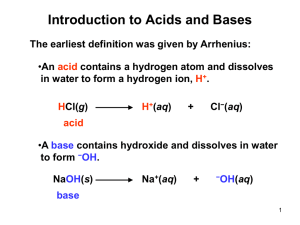
CHAPTER 8 – ACIDS & BASES Section 8.1-Understanding Acids & Bases Properties of Pure & Aqueous Substances Substance Most molecular compounds Most Ionic compounds Acids Bases Conductivity Liquid Solid Liquid Aqueous Aqueous No No No No Effect No No No Yes No Yes Yes Yes Yes No Effect Blue-Red Red-Blue NaOH(s)-Na+(aq) + OH-(aq) …..similar to ionic compounds acids in liquids are still showing acid’s molecular nature something happens when a solid acid is dissolved in water i.e. HCl(g) -- H+(aq) + Cl-(aq) Arrhenius hypothesized that bases will dissociate into individual positive & negative ions e.g. Ba(OH)2(s) ---> Ba+2(aq) + 2OH-1(aq) Arrhenius also theorized that acids first dissolve as individual molecules and then ionize into H+ and X- in solution e.g. HCl(g) -- H+(aq) + Cl-(aq) Sample Problem 1 Q: Write dissociation or ionization equation equations (as appropriate) for the following chemicals dissolving in water. Label each equation as either dissociation or ionization; a) potassium chloride (a salt substitute) b) hydroiodic acid ( a strong acid) A: a) KCl(s) --K+(aq) + Cl-(aq) =dissociation b) HI(aq) -- H+(aq) + I-(aq) =ionization REMEMBER: Ionic substances dissociate, acids ionize! acids as pure substances are molecular compounds and may be solid, liquid, or gas examples; HCl(g) or HCl(aq)-- H+(aq) + Cl-(aq) = gaseous acid HC2H3O2(l) or HC2H3O2(aq) -- H+(aq) + C2H3O2-(aq) =liquid acid H2C2O4(l) or H2C2O4(aq) -- H+(aq) + HC2O4-(aq) =solid acid Strong and Weak Acids acids can be classified as strong or weak Strong acids – have high electrical conductivity which is due to their High percentage ionization >99% e.g. H2SO4(aq), HNO3(aq), HCl(aq) most other acids have low electrical conductivity due to low percentage ionization and are called weak acids to communicate the percentage ionization and strength of an acid, we can write the percentage ionization over the chemical reaction arrow i.e. strong acids are usually >99% weak acids ionize <50% >99% HNO3(aq) ------- H+(aq) + NO3-(aq) =strong acid <50% HC2H3O2(aq) ------ H+(aq) + C2H3O2-(aq) =weak acid Sample Problem 2 Q: The following acidic solutions were tested (at equal concentrations and temperature) for electrical conductivity. Write ionization equations to explain the relative conductivity of each acid. a) hydrobromic acid (aqueous hydrogen bromide): high conductivity b) hydrofluoric acid (aqueous hydrogen fluoride): low conductivity >99% A: HBr(aq) ------- H+(aq) + Br-(aq) <50% HF(aq) ------- H+(aq) + F-(aq) Strong Bases: XOH(s) -- X+(aq) + OH-(aq) (100% dissociated as ions) *assume all bases are ionic hydroxides, all of which are strong bases Finish questions 1-6 on page 367. 8.2 pH of a Solution pH is a way of indicating the concentration of hydrogen ions [H+] in a solution according to Arrhenius’ theory H+ are responsible for the properties of acids the higher the concentration of H+ the more acidic the solution will be pure water would not be expected to contain any H+ or OH- at all careful testing has shown that 2 out of every billion water molecules will break down into; H2O(l)---- H+(aq) + OH-(aq) this will give a H+ concentration of 1 x 10-7mol/L therefore in a neutral solution [H+(aq)] = 1 x 10-7mol/L in an acidic solution [H+(aq)] > 1 x 10-7mol/L in a basic solution [H+(aq)] < 1 x 10-7mol/L this lead to the development of the pH scale the pH of a solution is defined as the negative of the exponent to the base 10 of the H+(aq) concentration (expressed as mol/L) examples: if [H+(aq)] is 10-7mol/L, the pH is 7 or if pH =2, the [H+(aq)] is 10-2mol/L Sample Problem 1 Q: What is the pH of (a) 1 x 10-2 mol/L [H+(aq)] in vinegar (b) [H+(aq)] =1.0 x 10-12mol/L in ammonia A: (a) pH=2 (b) pH=12.00 Do questions 1-6 on page 371. Sample Problem 2 Q: What is the [H+(aq)] of; (a) a carbonated beverage with a pH of 3.0 (b) an antacid solution for which the pH is 10.00 A: (a) [H+(aq)] = 1 x 10-3mol/L (b) [H+(aq)] = 1.0 x 10-10mol/L measuring pH of a solution can be done using pH indicators or pH paper to estimate the value for more precise measurements one would normally use a pH meter these meters work like small electric cells in which the electricity produced depends on the acidity of the solution Calculating pH and [H+(aq)] the relationship between [H+(aq)] and pH is easy to calculate if the concentration is only a power of 10 i.e. 10-3mol/L where pH=3 or [H+(aq)] =1 x 10-7mol/L where pH=7.0 But what if [H+(aq)] =2.7 x 10-3mol/L? The logarithm of a number is the exponent when the number is written in exponential form 100=102 0.001=10-3 log10 (102)=2 log10 (10-3)=-3 in general, if Y=10X, then log10(Y)=X fortunately we can use calculators to solve these problems. because [H+(aq)] are usually <0.1mol/L we define pH as the negative logarithm to avoid having almost all pH’s as negative numbers. i.e. pH=log10 [H+(aq)] the digits preceding the decimal point in a pH value are determined by the digits in the exponent of the [H+(aq)] these digits locate the position of the decimal point in the concentration value not the certainty of the value the number of digits following the decimal point in the pH value is equal to the number of significant digits expressing the certainty of the [H+(aq)] Example: [H+(aq)] =2.7 x 10-3mol/L pH=2.57 a) note 2 significant digits-----2 decimal places b) 3 in 2.7 x 10-3 only indicates where the decimal point goes c) .57 in pH indicates a certainty of 2 decimal places d) 2 in pH indicates where the decimal place will go Sample Problem 3 Q: An antacid solution has a hydrogen concentration of 4.7 x 10-11 mol/L. What is its pH? A: pH=-log10 [H+(aq)] =-log(4.7 x 10-11) pH=10.33 4 1 1 . 7 +/- Exp = log +/- OR - Exp +/- log 4 1 1 . = we may have to convert a pH reading to the molar concentration of [H+(aq)] therefore pH becomes the exponent i.e. [H+(aq)] =10-pH 7 Sample Problem 4 Q: The pH reading of a solution is 10.33. What is its hydrogen ion concentration? Be sure to indicate your answer with the correct certainty. A: [H+(aq)] =10-pH =10-10.33mol/L = 4.7 x 10-11mol/L 1 0 +/- . 3 Either OR 3 Inv 2nd log log OR depending on your calculator 10 YX 10.33 +/- XY ^ Summary pH is the negative power of 10 of the [H+(aq)] pH=-log[H+(aq)] or [H+(aq)] =10-pH solution: [H+(aq)] : pH : Acidic Neutral >10-7 <7 107 7 Basic <10-7 >7 * the higher the hydrogen ion molar concentration, the lower the pH. Do questions 8-10 on page 374. = Read section 8.3 on page 376. 8.4 Acid-Base Theories Arrhenius Concept of Acids & Bases To summarize Arrhenius’ definitions; Acid-- H+(aq) + an anion Base-- a cation + OH- Revision of Arrhenius’ Definitions The following cannot be explained by our original definitions; 1. compounds of hydrogen polyatomic ions e.g. NaHCO3(aq) & NaHSO4(aq) 2. oxides of metals & nonmetals e.g. CaO(aq) & CO2(g) 3. compounds that are neither oxides nor hydroxides e.g. NH3(aq) & Na2CO3(aq) but are still bases 4. compounds that contain no hydrogen but are acids e.g. Al(NO3)3(aq) revised theory looks at collisions with water molecules and the nature of the hydrogen ion Canadian scientist, Paul Giguere, has shown that there are hydrated protons e.g. hydronium ion H3O+ using H3O+(aq) to explain the formation of acidic solutions by strong acids such as HCl(g) is represented as; >99% HCl(g) + H2O(l) ------- H3O+(aq) + Cl-1(aq) for weak acids; 1.3% HC2H3O2(aq) + H2O(l) ------ H3O+(aq) + C2H3O2-(aq) (acetic acid) * in general, acidic solutions form when substances react with water to form hydronium ions Strong & Weak Bases strong bases dissociate to increase the concentration of OH-(aq) in an aqueous solution evidence indicates that all ionic hydroxides are all strong bases a strong base, like Ba(OH)2(s) will dissociate completely i.e. Ba(OH)2(s) -----Ba2+(aq) + 2OH-1(aq) and we don’t have to worry about water being present or needed for a weak base, like ammonia (NH3) we would need water i.e. <50% NH3(aq) +H2O(l) ------OH-(aq) + NH4-(aq) what about Na2CO3? Na2CO3(s) ---- 2Na+(aq) + CO3-2(aq) ( no H+(aq), no H3O+(aq), no OH-(aq)) <50% but CO32-(aq) + H2O(l) ------ OH-(aq) + HCO3-1(aq) Sample Problem 1 Q: A forensic technician tested the pH of a sodium cyanide solution and found that it had a pH greater than 7. Explain this evidence using chemical equations. A: NaCN(s) ---Na+(aq) + CN-(aq) <50% CN-(aq) + H2O(l) ----- OH-(aq) + HCN(aq) Questions 12-16 on page 386. The BrØnsted-Lowry Concept BrØnsted (Denmark) & Lowry (England) focused on the role of acids & bases in a reaction ACID: HCl(aq) + H2O(l) ------ H3O+(aq) + Cl-(aq) BASE: NH3(aq) + H2O(l) ------ OH-(aq) + NH4+(aq) water does not have to be a reactant e.g. H3O+(aq) + NH3(aq) ---- H2O(l) + NH4+(aq) water does not even have to be present. e.g. HCl(g) + NH3(g) ---- NH4Cl(s) acids and bases using this theory are called BrØnsted – Lowry acids or BrØnsted – Lowry bases BUT only for specific reactions i.e. a substance can be a BrØnsted – Lowry acid in one reaction and a BrØnsted – Lowry base in another a substance that appears as a BrØnsted – Lowry acid in one reaction and a BrØnsted – Lowry base in another is called amphiprotic. e.g. HCO3-(aq) in baking soda all hydrogen polyatomic ions are amphiprotic (sometimes called amphoteric) e.g. HCO3-(aq) + H3O+(aq) ----H2CO3(aq) + H2O(l) (neutralizes a strong acid) HCO3-(aq) + OH-(aq) ---- CO32-(aq) + H2O(l) (neutralizes a strong base) according to the BrØnsted – Lowry concept acid-base reactions involve the transfer of a proton so that the products formed in these reactions must differ from the reactants by a proton. e.g. HC2H3O2(aq) + H2O(l) ---- H3O+(aq) + C2H3O2-(aq) - a product formed as a result of an acid losing a proton is called a conjugate base. e.g. C2H3O2-(aq) - a product formed as a result of a base gaining a proton is called a conjugate acid. e.g. H3O+(aq) - a pair of substances that differ only by a proton is called a conjugate acid-base pair. HC2H3O2(aq) + H2O(l) ---- H3O+(aq) + C2H3O2-(aq) Questions 1-11 on page 392. 8.5 Acid-Base Reactions active metal + acid---- H2(g) + ionic compound e.g. Mg(s) + 2HCl(aq) --- H2(g) + MgCl2(aq) to clean up spills, emergency response teams use the knowledge that all acids can react with carbonates (Na2CO3(aq) and CaCO3(s)) to form carbonic acid, H2CO3(aq) carbonic acid is unstable and can quickly form CO2 and H2O so therefore an acid + a carbonate---- H2CO3(aq) + an ionic compound --- CO2(g) + H2O(l) + an ionic compound e.g. 2HNO3(aq) + Na2CO3(aq) ---- CO2(g) + H2O(l) + 2NaNO3(aq) lastly, acids can react with bases in a neutralization reaction i.e. HCl(aq) + KOH(aq) ----- H2O(l) + KCl(aq) H+(aq) + Cl-(aq) + K+(aq) + OH-(aq) -- H2O(l) + K+(aq) + Cl-(aq) (total ionic equation) H+(aq) + OH-(aq) --- H2O(l) (net ionic equation) or H3O+(aq) + OH-(aq) --- 2H2O(l) (net ionic equation) precipitation reaction i.e. acid + ionic compound -- precipitate + acid e.g. 2HI(aq) + Pb(NO3)2(aq) -- PbI2(s) + 2HNO3(aq) can be used to determine the initial concentration of HI(aq) Acid-Base Titration Titration – is an important and common technique used to determine the concentration of substances in solution known volume of a sample is put in an Erlenmeyer flask Titrant – (solution in the buret) is added slowly to the sample in the flask an indicator is used to tell us when the reaction ends the point at which the indicator changes colour is called the end point you must know the concentration of one of the reactants (standard solution) Titration Requirements 1. 2. 3. 4. spontaneous-chemicals should react without adding extra energy fast- should react quickly quantitative- more than 99% complete stoichiometric-single whole number mole ratio of reactants & products Example: Titration of Hydrochloric Acid -Take 1.59g Na2CO3(s) to make 100.0mL of stock solution -Samples (10.00mL)of stock solution are taken and titrated with HCl(aq) which has been diluted by a factor of 10 Results: TRIAL Final Buret Reading (mL) Initial Buret Reading (mL) Volume of HCl Added (mL) Calculate C of Na2CO3(s) VNa2CO3= 100mL MNa2CO3=105.99g/mol mNa2CO3=1.59g 1 13.3 0.2 13.1 2 26.0 13.3 12.7 3 38.8 26.0 12.8 4 13.4 0.6 12.8 1st step: Calculate n of Na2CO3(s) using n=m/M n=1.59g/105.99g/mol =0.0150mol 2nd step: CNa2CO3=n/v =0.0150mol/0.10000L =0.150mol/L step 3: use balanced equation 2 HCl(aq) + Na2CO3(aq)-----H2CO3(aq) + 2NaCl(aq) VAV=12.8mL V=10.00mL C=? C=0.150mol/L Note: we are using 10.00mL not 100.00mL NNa2CO3=10.00mL x 0.150mol/L =1.50mmol nHCl=1.50mmol x 2/1 = 3.00mmol CHCl=n/v 3.00mmol/12.8mL =0.234mol/L Therefore the molar concentration of the HCl was 0.234mol/L Sample Problem 1 Q: An acid rain sample containing sulphurous acid was analyzed in a laboratory using a titration with a standard solution of sodium hydroxide. Use the evidence given to determine the concentration of the sulphurous acid. Titration of 25.0mL of H2SO3(aq) with 0.105mol/L NaOH(aq) TRIAL Final Buret Reading (mL) Initial Buret Reading (mL) Volume of HCl Added (mL) 1 11.1 0.3 10.8 2 21.7 11.1 10.6 3 32.4 21.7 10.7 A: H2SO3(aq) + 2 NaOH(aq) --- Na2SO3(aq) + 2H2O(l) V=25.0mL V=10.7mL C=? C=0.105mol/L nNaOH = 10.7mL x 0.105mol/L = 1.12mmol nH2SO3 = 1.12mmol x 1/2 = 0.562mmol CH2SO3 = n/V = 0.562mmol/25.0mL = 0.0225mol/L Therefore the molar concentration of the H2SO3(aq) was 0.0225mol/L