Ka, Kb, Kw: Acid-Base Chemistry Lecture Notes
advertisement
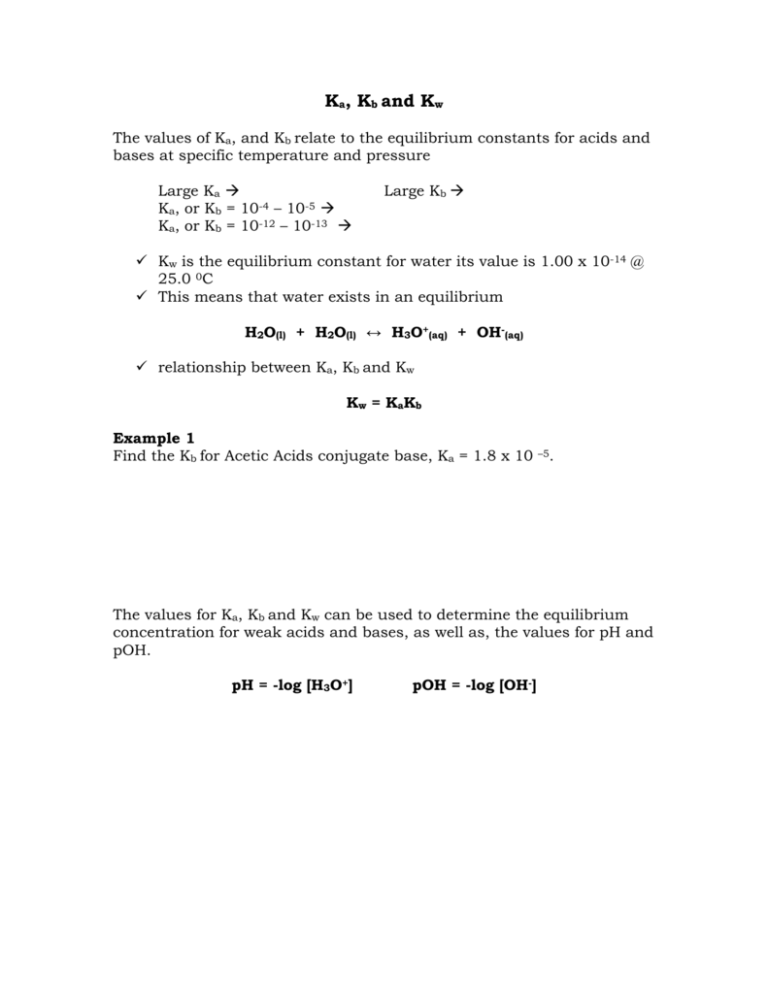
Ka, Kb and Kw The values of Ka, and Kb relate to the equilibrium constants for acids and bases at specific temperature and pressure Large Ka Ka, or Kb = 10-4 – 10-5 Ka, or Kb = 10-12 – 10-13 Large Kb Kw is the equilibrium constant for water its value is 1.00 x 10-14 @ 25.0 0C This means that water exists in an equilibrium H2O(l) + H2O(l) ↔ H3O+(aq) + OH-(aq) relationship between Ka, Kb and Kw Kw = KaKb Example 1 Find the Kb for Acetic Acids conjugate base, Ka = 1.8 x 10 –5. The values for Ka, Kb and Kw can be used to determine the equilibrium concentration for weak acids and bases, as well as, the values for pH and pOH. pH = -log [H3O+] pOH = -log [OH-] Example 2 Acetic Acid is a weak acid with a Ka = 1.8 x 10 –5 @ 25 0 C. If given a solution of 1.00M determine the pH, pOH and the % disassociation. % disassociation = # of H+ or OH- moles disassociated # of moles initially present Example 3 Calculate the Ka of hydrofluoric acid, HF(aq), if a 0.100 mol/L solution at equilibrium at SATP has a percent ionization of 7.8%. Example 4 Hydrazine, a component of Rocket Fuel, is a weak base that has a Kb of 9.6 x 10-7. What is the pH of a 4.56M solution of hydrazine? pH of Salt Solutions Salt - a complex composed of anions and cations - the force of attraction between the positive (cation) and negative (anion) charges allow the complex to exist. Acids - have the general form HA - when placed into water they form H30+(aq)/H+ ions and Aions Example 1 HCl HBr H2SO4 Bases - have the general formula: 1 BOH 2 BNH - when placed in water they form: 1 H2O(l) + BOH(aq) B+(aq) + OH-(aq) 2 H2O(l) + BNH(aq) BNH +(aq) + OH-(aq) Example 2 KOH H2O(l) + NH3(aq) The anions from the acids and the cations from the bases can be used/found in salts Example of Acid Anion Salts (Conjugate Bases)











