Catalysis: Heterogeneous & Homogeneous Catalysts Explained
advertisement
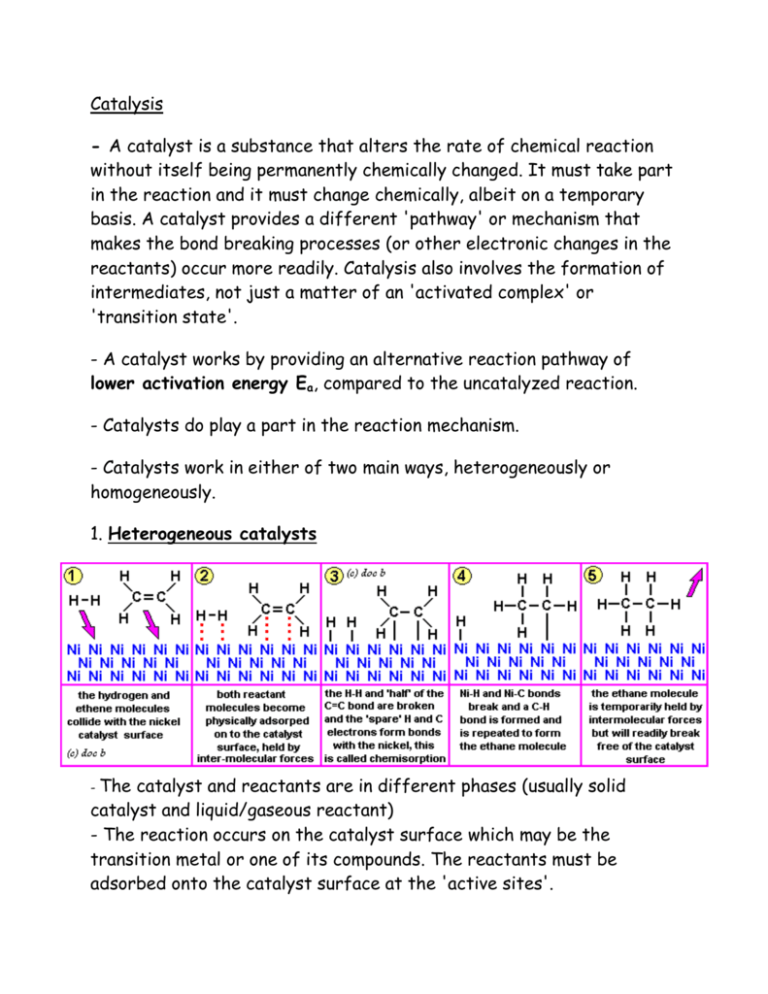
Catalysis - A catalyst is a substance that alters the rate of chemical reaction without itself being permanently chemically changed. It must take part in the reaction and it must change chemically, albeit on a temporary basis. A catalyst provides a different 'pathway' or mechanism that makes the bond breaking processes (or other electronic changes in the reactants) occur more readily. Catalysis also involves the formation of intermediates, not just a matter of an 'activated complex' or 'transition state'. - A catalyst works by providing an alternative reaction pathway of lower activation energy Ea, compared to the uncatalyzed reaction. - Catalysts do play a part in the reaction mechanism. - Catalysts work in either of two main ways, heterogeneously or homogeneously. 1. Heterogeneous catalysts - The catalyst and reactants are in different phases (usually solid catalyst and liquid/gaseous reactant) - The reaction occurs on the catalyst surface which may be the transition metal or one of its compounds. The reactants must be adsorbed onto the catalyst surface at the 'active sites'. - This can be physical adsorbed or 'weakly' chemically bonded (chemisorption/adsorption) to the catalyst surface. Either way, it has the effect of concentrating the reactants close to each other and weakening the original intra-molecular bonds within the reactant molecules and so allows a greater chance of 'fruitful' collision. - The diagram above illustrates a typical heterogeneous catalysis e.g. hydrogenation of alkenes with hydrogen and a nickel catalyst. - In catalytic converters the very expensive metals Pt, Rh and Pd are used. Cu and Ni are cheaper alternatives but they are vulnerable to catalytic poisoning of the active sites by traces of sulphur dioxide in the exhaust gases. Once poisoned, the catalyst in a converter cannot be regenerated and its a new costly converter! - The purpose of a catalytic converter is to convert harmful NO and CO gases to less harmful N2 and CO2 2. Homogeneous Catalysts - The catalyst and reactants are in the same phase (usually a solution), and so the catalyzed reaction can happen throughout the bulk of the reaction medium. - Catalysis can be due to temporary changes in the oxidation state and ligand(s) of a transition metal ion and results in a 'catalytic cycle'. In other words, the reaction occurs via some intermediate species and the original catalyst is reformed.