Homework #1- Molecular Compounds and Ions
advertisement

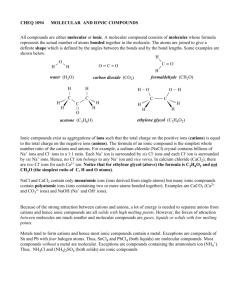
Name _______________________ Date ________________________ Homework #1- Molecular Compounds and Ions Completion 1. every substance is either an element or a(n) ___________________. A compound is either __________________ or ionic in nature. Molecular compounds are composed of two or more __________________. 2. The representative particle of a molecular compound is a _________________. Ionic compounds are composed of oppositely charged _____________ combined in electrically neutral groupings. 3. Molecular compounds tend to have _______________ melting and boiling points, while ionic compounds tend to have _______________ melting and boiling points. Ions form when atoms or groups of atoms ____________ or ___________ electrons. True-False (State whether each is true or false and give a justification below as to why or why not) _____________4. An anion is any atom or group of atoms with a positive charge. _____________ 5. For metallic ions, the name of the ion is the same as the name of the element. _____________ 6. For ions of nonmetals, the name of the ion is the same as the name of the element. _____________ 7. A molecule contains two atoms Matching (Match each description in Column B to the correct term in Column A) Column A ___ 8. molecule ___ 9. molecular compounds ___ 10. ions ___ 11. cations ___ 12. anions ___ 13. ionic compounds Column B a. compounds composed of molecules b. atoms or groups of atoms with a negative charge c. compounds composed of cations and anions d. atoms or groups of atoms that have a positive or negative charge e. smallest electrically neutral unit of a substance that retains the properties of the substance 14. State the number of electrons lost or gained in forming each of these ions. a. Mg2+ _________________ c. Br - ____________________ b. Ca2+ _________________ d. Ag+ ___________________ 15. Name each ion and tell whether it is an anion or cation. a. Mg2+ _________________ c. Br - ____________________ b. Ca2+ _________________ d. Ag+ ___________________ 16. Classify each particle as an atom, cation, anion, or molecule. a. Fe3+ _______________ f. O2- ________________ b. F- g. Na+ ________________ _______________ c. CH4 _______________ h. He ________________ d. Ne _______________ i. CO2 ________________ e. O2 _______________ j. Ca2+ ________________