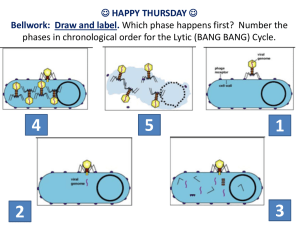
Transduction of mammalian cells using lentiviral vectors
advertisement

GM Ref. : 11/22 Newcastle University Microbiological Hazards and Genetic Modification Safety Advisory Sub-Committee 2 Proposal and Risk Assessment for Categorisation of a Procedure Involving Work with Genetically Modified Human and Animal Viruses and Viral Vectors Please complete the form by computer and send it as an email attachment in rtf format to your School GM Chair. The principal investigator and School GM Chair should sign the declaration page of the electronic copy of the form. After review and approval, the School GM Chair will send the form as an email attachment in rtf format to the University Biological Safety Officer. Please Note: Principal investigators must send all GM risk assessment forms to the School GM Chair and not to the University Biological Safety Officer. Guidance on completing this form is provided in the GM Risk Assessment section of the University Safety Office website. Title of project Principal investigator Transduction of mammalian cells using lentiviral vectors Prof A N Other Date of application Location of work Today Medical School (Building & Room. No.) Summary Details (Delete or enter Not Applicable (N/A) where appropriate) 2 GM activity Class Connected programme? (Class 2 or above) No Likely completion date 12/2030 If Yes, and not Parent, give Parent GM Ref. Section 1 Personnel 1.1: Briefly indicate your experience of working with microorganisms and genetically modified organisms and any training you have received PIs or project leaders training and background should be detailed here 1.2.1: Other workers on the project (if known) 1.2.2: Qualifications 1.2.3: Experience / Training Full list of staff to work on the project GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 1 of 8 GM Ref. : 11/22 Section 2 Project 2.1: Description of the project, including the methods to be used and the purpose of the genetic modification This risk assessment covers research projects in our lab utilising lentivirus vectors and mammalian host cells. The projects involve the assessing the biological function of candidate genes implicated in malignant progression which emerge from ongoing genomics projects characterising collections of primary tumour material ). Lentiviral particles will be generated on site containing either a cDNA or appropriate shRNA. cDNAs may occasionally contain a mutation where a dominant negative or constitutively active version of the gene is required. The purpose is to perturb the natural state of the gene in question and to assess the effects on cells. Downstream analysis may involve cell cycle analysis, proliferation, cytotoxicity, apoptosis or differentiation assays, and development of in vivo xenograft models. RNA, DNA and protein would typically be extracted from infected cells in each case to verify appropriate infection and to validate cDNA/shRNA expression. A lentiviral pool expressing various cDNAs or shRNAs may be used to create a pool of cells with several integrated constructs which will then undergo in vitro or in vivo selection to screen for constructs which either promote or inhibit leukemic cell proliferation or engraftment. 2.2: Will you cultivate on a large scale (e.g. 10 or more litres per culture) 2.3: Host organism No Cell lines in culture: 293T Human embryonic kidney cell line (packaging cell line), adherent and suspension human and mammalian cell lines (including cancer cells) (such as DAOY, MON, KD, Molt-4, RPMI-8402, MN-60, Pre-B-697) immortalised normal cells and primary mammalian cells. 2.4: Vector system We will be using second and subsequent generation lentivirus. They are replication incompetent and self inactivating (SIN). The systems separate the packaging signals and viral LTRs on the expression plasmid from the viral structural and expression genes (gag, pol and rev from FIV or HIV and viral envelope glycoproteins such as the VSV-G gene from Vesicular Stomatitis Virus, in place of HIV or FIV env). The viral structural and expression genes and the envelope glycoprotein genes are separated on at least two additional plasmids. 2.5: Origins, nature of modification(s), and intended function of the genetic material involved cDNA sequences of humans or mice origin and/or the appropriate shRNA sequences will be cloned into the lentiviral vectors. These may include partial or entire gene sequences, or mutant forms of the gene sequences, under the control of constitutive promoters. Many of the genes or targets of the shRNAs will have unknown function but may include known oncogenes or tumour suppressors. (examples should be included..) The aim is to use either overexpression or knock-down of gene-expression to assess the potential contribution to tumourigenesis of each candidate identified from our genomics screens. Once a stably infected cell line is produced experiments performed will depend on the particular gene selected but will typically involve a panel of typical proliferation, apoptosis and/or invasion assays as well as collection of RNA/DNA/Protein for further profiling analysis and potentially in vivo engraftment. GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 2 of 8 GM Ref. : 11/22 Section 3 Risk Assessment NB : This section should include the justifications for any assessments made and sufficient detail to support them. Level of risk may be estimated using the matrix given at the end of this form. 3A Risk to Human Health 3A.1: Characteristics of the host, virus or viral vector and any hazards associated with it 3A.1.1: Describe all hosts that will be used, including where relevant, bacterial hosts and packaging cell lines used to produce non-replicating viral particles Packaging cell line 293T (and thereof derived cell lines) is derived from the well-characterised 293 cell line (Pear et al., PNAS 1993; 80; 8392; ATCC CRL-11268). This cell line stably and constitutively expresses a temperature-sensitive version of the SV40 large T antigen. This cell line will be used to produce lentiviral particles. These vectors alone are replication incompetent owing to their lack of envelope and gag-polymerase encoding sequences. Plasmid DNA will be produced in standard K12 E-coli hosts but mammalian promoters would preclude expression of ORFs. Mammalian cell lines and primary mammalian cells will be used for infection with the supernatant generated by the packaging cells. Cell lines such as MT2 or MT4 producing infectious particles will not be used for transduction. Most cell lines are derived from solid tumours or leukaemias. All cell lines have a long history of safe use and are routinely handled in UK labs at CL2. Yes 3A.1.2: Is the viral vector disabled If Yes, How Deletion of accessory genes vpr, vpu, vif and nef mean that the vector is unable to replicate once it has transduced the target cell. In addition third generation lentivirus are deleted for the tat gene and carry a SIN deletion of the 3’ LTR (U3) which results in “selfinactivation” of the lentivirus following transduction of the target cell, precluding adventitious activation of the vector by endogenous retroviruses and minimising the risk of recombination with ERVs. 3A.1.3: Describe the origin of the virus, the mechanism of attenuation, and its stability in both the parent viral vector and the recombinant vector The transfer vector systems are derived from FIV or HIV and have been specifically engineered for biosafety by separating the packaging signals and viral LTR’s on the expression plasmid from the viral structural and expression genes (gag, pol and rev from FIV and the VSV-G gene from Vesicular Stomatitis Virus, or similar alternatives, in place of FIV or HIV env) encoded on three or four separate plasmids, which remain in the packaging cell line, effectively precluding the production of replication competent virus in the target cell or should the viral vector escape containment. FIV vectors are included as they may be more efficient for some cell types. It does not imply any different containment or risk considerations than HIV based vectors. The plasmids expressing these gene products carry no packaging signals or LTRs and so cannot themselves be mobilised with the vector and have been engineered not to contain any regions of homology to each other or to the viral vector, to prevent undesirable recombination events which might result in replication competent virus being produced. The viral vector and recombinant virus have a broad host range when pseudotyped with e.g. the VSV-G envelope protein. The normal tropisms of HIV and FIV have been broadened to include multiple tissues from a broad range of species. Pseudotyping with alternatives to VSV-G, for example measles virus derived glycoproteins, will produce particles with more restricted tropisms. 3A.1.4: Indicate the probability of reversion to the wild type Reversion to wild type is extremely unlikely given that several recombination events would be needed reconstitute an active viral genome, the viral genes are present on three different plasmids which have minimal sequence homology and the viruses are selfinactivating following insertion (i.e. the LTR’s are destroyed upon genome insertion). No 3A.1.5: Is the viral vector replication competent Yes 3A.1.6: Are all potential routes of transmission of the virus known, eg those that may occur during a laboratory accident 3A.1.7: If Yes, will the routes of transmission deliver the virus or its products to tissues where it may be biologically active The viral vector would be able to transduce many tissues should it come in contact with them. The major hazard is therefore represented by the packaged virus prior to infection of the target cells and residual virus in the medium of infected cells. The two potential transmission routes are by external exposure (either skin lesions or mucous membranes; only in the case of very high titres and aerosol production) and by accidental injection/inoculation using sharps. No 3A.1.8: Is there a potential for the transmission of the naked nucleic acid Yes 3A.1.9: Does the viral vector infect humans or human cells in vitro Level of risk Low. Severity of harm Minor given that infection of human tissue if achieved would be with non-replicable virus and low-efficiency. Likelihood of harm Low given lab procedures for avoiding contamination, in particular no sharps, etc. The virus is generally weak and does not survive long outside of buffered solutions. Infection requires optimal conditions and the use of additives in cell culture. Immune-competent mice show sterilising immunity with wild-type virus infection; humans would also show such sterilising immunity. Risk is GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 3 of 8 GM Ref. : 11/22 generally speaking no greater than that of handling human tissue or bodily fluids i.e. relatively safe given that proper precautions are taken to avoid infection see specialised training details 1.2.1 and 4.2. 3A.2: Source and characteristics of the inserted gene product and any hazards arising directly from its use 3A.2.1: Describe the nature of the inserted genes and the properties of the final genetically modified viral vector Known oncogenes or shRNAs that will silence known tumour suppressor genes may be inserted into primary cells and cell lines. There is therefore a theoretical possibility that they could render the cells neoplastic. If during an unlikely event these cells were inoculated into the human organism, then these cells are recognised as non-self and will be identified by the immune system. Inserted gene products will be both genomic, cDNA sequences or small interfering double stranded RNA sequences originating in humans or rodents, including those which could be implicated in tumour progression, i.e. oncogenic and tumour suppressor DNA sequences. Specific functions will vary depending on the particular experiment, but will include functions that are known to contribute to the maintenance of pluripotency and / or differentiation and the regulation of cell growth and apoptosis. The gene products will be biologically active when transfected into mammalian cells and probably expressed at a level higher than seen in the wild type cells. However, the recipient transfected cells will be either primary human or mouse cells or well characterised stable eukaryotic cell lines in tissue culture that have a history of safe use and these hosts can be considered disabled hosts because the cells are unable to colonise the worker (i.e. not their own cells). Two potential risks to humans may arise from the hazards of working with the naked plasmid vector + insert DNA containing the potentially oncogenic DNA and the supernatant containing the viral particles from the packaging cell line (see 3.A.1.7). However, the transfection efficiency even under optimal culture conditions is negligible, and therefore transfection of researcher’s cells with naked DNA under accidental/non-optimal conditions would be effectively zero. The potential oncogenes will only be sufficiently oncogenic to induce tumourigenicity in immortalised cell lines or in premalignant cells or give a growth advantage to cells in culture without inducing tumourigenicity. Tumourigenesis is a multistage process requiring the interaction of many mutated genes which also lessens the risk from delivery of a single oncogene or tumour suppressor gene to a target tissue. The principle route of transmission that could lead to transduction of laboratory workers by packaged viral particles is inoculation. Therefore, use of sharps will be prohibited. Other possible routes of transmission such as inhalation, ingestion and eye splashes are less likely to lead to tumourigenesis because of the required very high titres, which will not be achieved under the given experimental conditions. Consequently, the risk of tumourigenesis is negligible. Yes 3A.2.2: Does the insert code for a protein with known or suspected physiological, pathological and or pharmacological effect 3A.2.3: Will the viral vector contain any natural or inserted oncogene and/or oncogenic sequences Level of risk Yes Low Severity of Harm (Low), Likelihood of Harm (Low) 3A.3: Hazards arising from the alteration of any existing pathogenic traits 3A.3.1: Is there reason to suspect that the tissue tropism or host range of the recombinant virus will be any different from that of the parent vector or virus 3A.3.2: Is there reason to suspect that the recombinant virus may have altered susceptibility to host defence mechanisms eg Will normal immune status be compromised by the recombinant virus eg Will vaccination protect against the recombinant virus No No No N/A No 3A.3.3: Is the recombinant virus likely to have any effect upon an immunocompromised host beyond those normally expected with the parental host No 3A.3.4: Will viral susceptibility to anti-viral drugs (if available) be affected by the genetic modification No 3A.3.5: Could the route of transmission of the recombinant virus be altered 3A.3.6: If Yes, what are the predicted effects of the recombinant viruses in tissues which it would not normally infect [ENTER DETAILS HERE] Level of risk Negligible The particular insert used is not expected to alter any potential pathogenic traits of the virus 3A.4: Potential hazard of harmful sequences within the virus being transferred to related viruses Negligible since there are expected to be only very small regions of homology between the viral vector and wild-type viruses, GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 4 of 8 GM Ref. : 11/22 making recombination events unlikely. Level of risk Effectively zero 3A.5: The overall likelihood that, in the event of exposure, the GM virus could cause harm to human health The GM virus would have the ability to infect human tissues should it come in contact with them. The major hazard is therefore represented by the packaged virus prior to infection of the target cells and residual virus in the medium of transfected cells. The main potential transmission routes are by external exposure to high titres (either skin lesions or mucous membranes). Some of the genes that will be studied could have oncogenic effects. If those genes were to be transfected directly into living human tissue the detrimental effects that they could cause are uncertain but unlikely on their own to transform affected cells. Given that virus is replication incompetent and will only infect at low efficiency in sub optimal conditions any potential harm would be extremely limited. These risks will be further minimised by measures described in section 4. Level of risk low Assign the provisional containment level: (delete as appropriate) 2 3B Assessment for Environmental Harm Note : If the answer to a question is Yes, provide brief details 3B.1: What is the capacity of the GMM to survive, establish, disseminate with and or displace other organisms No 3B.1.1: Is there reason to suspect that the recombinant virus may have enhanced environmental survival factors; eg enhanced tolerance to UV, temperature, desiccation etc [ENTER DETAILS HERE] 3B.1.2: Are all potential routes of transmission or escape of the virus to the environment known eg following a laboratory accident Yes Viral particles capable of transducing a wide range of mammalian cells will be produced by the packaging cell line and these represent the hazardous material. However, because of its incompetence to replicate, short half-life and inefficient transduction rates, the risk of transmission outside the laboratory is effectively zero. No 3B.1.3: If Yes, will the recombinant virus or its products gain access to organisms in which effects may be manifested [ENTER DETAILS HERE] Level of risk Effectively zero 3B.2: What is its ability to cause harm to organisms other than humans 3B.2.1: Is the host pathogenic to organisms other than humans No [ENTER DETAILS HERE] 3B.2.2: Does the insert code for a protein with known or suspected inhibitory, detrimental, No or other physiologically active effect on any organisms other than humans [ENTER DETAILS HERE] 3B.2.3: Is there a potential for harmful effects of gene expression on other organisms No [ENTER DETAILS HERE] 3B.2.4: Will the recombinant virus alter infectivity or interactions with host defence mechanisms [ENTER DETAILS HERE] eg Will the normal status of host defence systems be compromised by the recombinant virus [ENTER DETAILS HERE] 3B.2.5: Is the recombinant virus likely to have enhanced effects on a weakened host or one lacking normal vigour beyond those normally expected with the parent virus No No No [ENTER DETAILS HERE] 3B.2.6: Will viral susceptibility to control agents be affected by genetic modification No [ENTER DETAILS HERE] 3B.2.7: Will the insert cause changes in the host range of the virus No [ENTER DETAILS HERE] 3B.2.8: Is there reason to suspect that the tissue tropism of the recombinant virus in host No GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 5 of 8 GM Ref. : 11/22 organisms will be different from that of the unmodified virus [ENTER DETAILS HERE] Level of risk Effectively zero 3B.3: What is the potential for transfer of genetic material between the GMM and other organisms The viral vectors are capable of transducing a wide range of mammalian cells; however it would not transfer genetic material to microbial organisms. Release of the viral vector into the environment is very unlikely due to the standard procedures employed in the tissue culture facility. Even should such an event occur the virus is replication incompetent and self inactivating, . It is highly unlikely that the genetically modified cell lines could transfer the viral vector into the environment since they have very limited ability to propagate outside of the cell culture facility, and no means to mobilise the transfected DNA sequences to other organisms. Level of risk Effectively zero 3C Final Activity Class Assign the final activity Class: (delete as appropriate) 2 Section 4 Control Measures to be Used 4: Provide details of the control measures to be used to protect human health and the environment and the means by which their use and effectiveness will be monitored. This must include details of the inactivation procedures to be employed for waste contaminated with GMM, the expected degree of kill and any appropriate validation procedures 4.1: Containment level 2 NOTE: While containment level 1 is sufficient to control risks associated with the GM work, in accordance with the SACGM Compendium of Guidance 2000, Part 2A, Annex VI, a parallel BioCOSHH assessment has considered hazards associate with possible adventitious agents when using mammalian cell culture. The BioCOSHH assessment concludes that containment level 2 should be used to control these hazards and consequently all work involving mammalian cell culture will be carried out under full containment level 2. This does not alter the assignment of the GM activity class. Established code of practice (SOP) for work at containment level 2 will be employed. 4.2: Controls In accordance with the SACGM Compendium of Guidance, GMM mammalian cells will be handled at containment level 2 and using microbiological safety cabinets. All GM virus waste will be properly inactivated by autoclaving. Spillages will be soaked with tissue paper, which will afterwards be autoclaved. Contaminated surfaces may be decontaminated with either a 1% virkon solution or 70% Ethanol. Only plastic pipettes will be used in the tissue culture facilities. Furthermore, all contaminated materials and media including waste destined for subsequent incineration will be autoclaved (see 4.3). The use of sharps will be prohibited and all precautions will be taken to identify procedures or devices that might be used in these projects which could cause an injury to the researchers. Where so identified safe alternative methods or equipment will be sought. The generation of aerosols will be avoided or contained. Given the uncertainty about the hazards of most potentially oncogenic sequences and the small quantities used (microlitre/microgram quantities), prevention of exposure or total enclosure is not “reasonably practicable”. Therefore the following measures are applied in the light of this GM risk assessment, will be used: (1) Good laboratory techniques will be strongly emphasised. Designated workers will be trained in good laboratory techniques before commencing work with potentially oncogenic DNA sequences. They will be made fully aware of the potential hazards of such work. (2) Access to the laboratory where novel DNA fragments which could potentially be oncogenic is handled, will be limited to authorised personnel and designated workers. (3) All experimental procedures involving naked DNA will be performed so as to minimise aerosol production. Procedures which are likely to generate aerosols such as the use of sonicators, vigorous shaking and mixing etc. will be avoided, or where necessary, will be carried out in closed containers or a class II microbiological safety cabinet. (4) Normal cell sorting will only be performed with cultures free of detectable infectious particles. Sorting of cultures containing detectable amounts of infectious particles will be performed in class II microbiological safety cabinets. GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 6 of 8 GM Ref. : 11/22 (5) Procedures using lentiviral particles, packaging cell lines and exposed cells will only be carried out in the tissue culture suite. Normal procedure for handling biological material in the TC suite will be largely sufficient for handling the cells and particles. Specific items of relevance here include (6) Safety cabinets are routinely disinfected prior to and following use (7) Gloves and lab coats to be worn throughout (8) Designated microbiological safety cabinets to be used (9) No wild type virus to be used in same hood (10) The packaging lentivirus will be monitored for their ability to produce infectious particles by taking supernatant from transduced cells and trying to infect cell lines. If the lentivirus are truly replication incompetent, no resistant clones will be generated. Staff competence will be checked during regular inspections of tissue culture facilities and during their project presentations involving lentiviral work. 4.3: Inactivation of genetically modified organisms All contaminated materials, including waste destined for incineration, will be inactivated by autoclaving (100% kill) prior to disposal of waste or cleaning and recycling of reusable laboratory equipment, such as glassware. Autoclaves will be validated by annual thermocouple mapping and each run will be monitored by continuous chart (or digital) recording of the temperature/time profile. Section 5 Emergency Planning 5: Are there risks to human health and safety or to the environment that require an emergency plan for action in case of accidental release No If Yes, provide full details of the plan [ENTER DETAILS HERE] School Declaration and Approval Signatures Title of project : School : Declaration : I declare that this work will be conducted in accordance with University rules, practices and requirements on GM procedures, and that if at any stage there is any indication that hazards and/or risks could be significantly higher than originally assessed, then the work will cease until such time the risk assessment has been revised, and approval granted from the School and/or University GM Safety Committee as appropriate. Principal investigator : As the principal investigator for this GM project you have a legal responsibility to ensure that all those involved/working on the project have an appropriate level of training and expertise to enable safe working. This includes ensuring that they read and understand this risk assessment, and that all procedures they undertake, including the control measures, are in strict accordance with those approved for the project. To ensure the latter you are advised to check for compliance with procedures from time to time, and make an appropriate record to be kept as part of the project file. Sign and date Declaration : Note Role (delete as appropriate) Print name Sign and date I declare that this risk assessment has been scrutinised and approved by the School GM Safety Committee. To be signed by the School GM Chair, or, in the event they are the principal investigator, by another member of the School GM Safety Committee. Chair GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 7 of 8 GM Ref. : 11/22 Risk Estimation Matrix Severity of harm Severe Moderate Minor Negligible Likelihood of harm High Medium Low Negligible High High Medium/low Effectively zero Medium Medium/low Low Effectively zero Effectively zero Effectively zero Effectively zero Effectively zero High Medium Low Effectively zero GM Risk Assessment Form 2 - Genetically Modified Human and Animal Viruses and Viral Vectors v3 Page 8 of 8