10 Harvesting Chemical Energy
advertisement

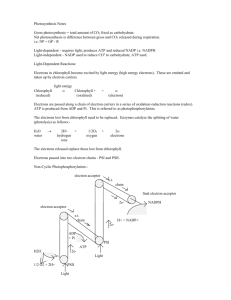
Harvesting Chemical Energy As open systems, cells require outside energy sources to perform cellular work. Only photosynthetic organisms have the ability to harness the energy from the sun. During Photosynthesis: CO2 and H2O are the raw materials used to make glucose Light energy is converted into chemical bond energy Chemical bond energy can be released to drive metabolic reactions by cellular respiration. Note: the chemical elements are recycled, but the energy is not! The storage and release of Free Energy The free energy is stored and transferred by ATP (adenosine triphosphate). Recall, the phosphate bonds have stored energy; breaking these bonds releases energy (to drive reactions); while forming new phosphate bonds temporarily stores the chemical energy. The compound receiving the phosphate group from ATP is said to be phosphorylated and becomes more reactive in the process. The phosphorylated compound loses its newly acquired phosphate group as work is performed. There are two types of phosphorylation: 1. Substrate level phosphorylation an enzyme cataslyzed reaction of Pi to ADP 2. oxidative phosphorylation forming of ATP directly through a series of enzyme catalyzed redox reactions where oxygen is the final electron acceptor (more about this in a bit). REDOX Reactions Also Release Energy Oxidation-reduction reactions = chemical reactions which involve a partial or complete transfer of electrons from one reactant to another; called «REDOX reactions» for short. 1. Oxidation = partial or complete loss of electrons 2. Reduction = partial or complete gain of electrons “LEO says GER” Loss of electrons = oxidation Gain of electrons = reduction The electron transfers require both a donor (becomes oxidized) and an acceptor (becomes reduced). Since electrons lose potential energy when they shift toward more electronegative atoms, redox reaction that move electrons closer to elements with a higher electronegativity (like oxygen) release energy. Cellular Respiration is a redox process that (ultimately) transfers hydrogen, including electrons with high potential energy, from sugar (glucose) to oxygen. Released energy is used to convert ADP to ATP. How is the energy transferred to a molecule of ATP? Essentially, glucose is broken down in a series of sequential steps so that the free energy released from breaking bonds can be harnessed (captured) bit by bit. Eventually this harnessed free energy is transferred to molecules of ATP as they are phosphorylated (i.e from ADP + Pi ATP) Why does the process need to occur in a sequence of steps? Activation energy = requires extreme heating to start combustion of glucose Exergonic reation = large amount of energy is released; would damage cells Therefore, the combustion of glucose occurs by a series of enzyme catalyzed steps, that result in: At each step of the way, hydrogen (along with electrons) are transferred to another substance (enzymes & coenzymes) When this happens, a portion of the reaction’s total energy is released and stored in ATP (ie. ATP is produced). An Overview of Cellular Respiration There are two types: Anaerobic respiration (fermentation) = ATP-producing pathway in which sugars are only partially degraded (low energy yield); proton donors and acceptors are organic molecules. Aerobic respiration = ATP-producing pathway in which sugar is fully oxidized (high energy yield); ultimate electron acceptor is oxygen (inorganic molecule) Overall Equation: (Aerobic) Aerobic Cellular Respiration occurs in four stages: 1. glycolysis 2. pyruvate oxidation (occurs only in eukaryotic cells) 3. Citric Acid or Kreb’s Cycle 4. Electron Transport Chain (ETC) Breaking down glucose to get H+ (and electrons) Using H+ (and electrons) by releasing them slowly trapping released energy in ATP Glucose + oxygen carbon dioxide + water + energy C6H12O6 + 6O2 6CO2 + 6H2O + 38 ATP (total) Stepwise fall of Electrons occurs due to special electron acceptors (NAD+ and FAD) and the electron transport chain. Hydrogen (and electrons) stripped from glucose is NOT transferred directly to oxygen, but are first passed to a special electron acceptor NAD+ NAD+ (nicotinamide adenine dinulceotide) and FAD (flavin adenine dinucleotide = are dinucleotides that function as a coenzyme in the redox reaction of metabolism. NAD+ and FAD are found in all cells assist enzymes in electron transfer during redox reactions What happens? During the oxidation of glucose, the electrons are transferred and temporarily trapped in NAD+ and FAD. This transferred is catalyzed by enzymes called dehydrogenases, which: o remove a pair of hydrogen atoms (2 electrons and 2 protons) from glucose (substrate) o deliver the two electrons (2e-) and one proton (H+) to NAD+ (or 2e- and 2H+ to FAD) o release the remaining proton (H+) into the surrounding solution. Overall reactions: The high energy electrons transferred to NAD+ and FAD are then passed down the electron transport chain to oxygen, powering the production of ATP by oxidative phosphorylation. Electron Transport Chains Consist of electron-carrier molecules built into cellular membranes Accept energy rich electrons from reduced coenzymes (NADH and FADH2) Since electrons lose potential energy when they are transferred to a more electronegative atom, this series of reactions releases energy. Release energy from energy-rich electrons in a controlled stepwise fashion powering the production of ATP Each successive carrier in the chain has a higher electronegative then the carrier before, so the electrons are pulled downhill towards oxygen (the molecule with the highest electronegativity), the final electron acceptor.