Chapter 10 Worksheet: Ions and Oxidation Numbers
advertisement

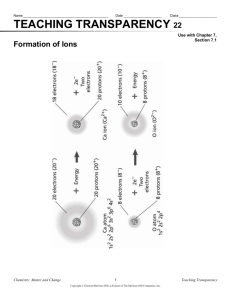
Chapter 10 Worksheet: Ions and Oxidation Numbers Name______________ 1. When an oxygen atom forms an oxide ion, O2-, it achieves a noble gas configuration. (a) What is a noble gas configuration A full outer level (b) What noble gas does oxide ion, O2-, have the electron configuration of? Neon 2. When an aluminum atom forms an aluminum ion, Al3+, it becomes more stable. (a) Are electrons lost or gained during this change? lost (b) How many electrons are lost/gained? Three electrons are lost. (c) Why is an aluminum ion, Al3+, more stable than an aluminum atom? Al = [Ne]3s23p1. If it loses 3 electrons it will have the same electron arrangement as neon giving it a full outer level which is more stable. 3. Why do most transition elements exhibit more than one possible oxidation number? Because they can lose electrons from different energy levels and do not have to lose them all at once but instead can lose one level at a time. 4. Nearly all transition elements can have oxidation numbers of 2+. Explain why? Most have 2 valence (outer level) electrons which they can lose. 5. The electron configuration of iron, Fe, is [Ar]4s23d6. (a) Write the electron configuration for Fe2+ [Ar]3d6 (b) Write the electron configuration for Fe3+ [Ar]3d5 (c) Explain why Fe3+ ion tends to form. This has a ½ full outer sublevel which is as stable as it can get since it cannot lose 8 electrons and be Fe8+. 6. What are the two most common oxidation numbers for titanium, Ti? 2+ and 4+ 7. Determine the oxidation number for each atom/ion within the following substances. (a) CaF2 Ca 2+, F - (b) Ag3P Ag+, P 3- (c) Sc2S3 Sc 3+, S 2- (d) CO C 2+, O 2- (e) CO32- C 4+, O 2- (f) H2O H +, O 2- (g) NaH Na+, H - (h) NH4+ N 3-, H + (i) BaSO4 Ba 2+, S 6+, O 2- (j) Cu(MnO4)2 Cu 2+, Mn 7+, O 2- (k) Cr(OH)4 Cr 4+, O 2-, H + (i) KCN K +, C 2+, N 3-