Mainly decomposition – generation of energy (mainly ATP)
advertisement

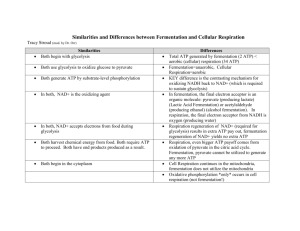
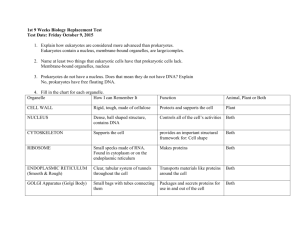
9. METABOLISM – C TURNOVER Two parts of metabolism – catabolism, anabolism Connection – by-products, energy Preferable source of energy for chemoorganotrophs – sugars 9.1. Catabolism Mainly decomposition – generation of energy (mainly ATP) polysaccharide (cellulose) hydrolysis monosaccharide glycolysis, E-D, hexose - P-pathway → pentose - P-pathway pyruvate fermentation respiration Glykolysis (Embden-Meyerhof pathway) Glucose Fosforylation +2ATP Fructose 1,6 bisphosphate 2 trioses forming glyceraldehyde 3-phosphate dihydroxyacetone phosphate 2 pyruvate + 2NADH + 2H2O + 4ATP fermentation (anaerobic) respiration (aerobic, anaerobic) Fermentation – donor, acceptor H+(e-) organic Ethanolic – Lactic – Butyric - Propionic Respiration - donor H+(e-) organic inorganic; acceptor H+(e-) inorganic, acceptor H+(e-) inorganic Generation ATP Catabolism = main source of energy for chemotrophs Fermentation – 2ATP Aerobic respiration – 38ATP (Escherichia coli synthesizes 2500000 molecules ATP per second) The use of ATP: ATP ADP + Pi + E Anabolism Nutrient uptake Movement Heat energy Luminescence (light energy) Electrical energy (electrical potential) 9.1.1. Fermentation Anaerobic pathway, energy usually only in the first step (glycolysis) Lactic acid fermentation (Pyruvate = acceptor of H+) Characteristics: mesophilic, aerobic – anaerobic, chemoorganotrophs, need for simple sugars Homolactic fermentation – major end product = lactic acid Typical pathway - EM Streptococcus – yoghurt, mouth Lactococcus – cheese, silage Enterococcus – gut, silage, probiotics Lactobacillus (some species) – cheese, yoghurt, silage, gut, vagina, probiotics Heterolactic fermentation – important end product = lactic acid; additional products (ethanol, CO2, acetic acid…) Typical pathway – pentose phosphate Leuconostoc – sauerkraut Lactobacillus (some species) – sauerkraut, gut [Bifidobacterium (60% acetic acid, 40% lactic acid) – gut (esp. breast fed babies), probiotics, yoghurt] Ethanolic fermentation Pyruvate after decarboxylation (-CO2) to acetaldehyde is reduced on ethanol glucose+2ADP+Pi 2ethanol+2CO2+2ATP Characteristics: anaerobiosis, simple sugars Saccharomyces – main yeast in ethanolic f. bakery, alcoholic beverages, vitamins Butyric fermentation Main products: butyric acid, butanol, acetone, CO2, H2 Additional products: other organic acids Characteristics: mesophilic – thermophilic, strictly anaerobic, different C-sources (simple and poly-sugars), different Nsources (for some N2) Clostridium – digestive tract, soil, sewage Methanogenesis Fermentation of acetate to methane (one of variants for methane production) CH3COO- + H+ CH4 + CO2 Typical for some chemoorganotrophic Archea Important for biogas production Propionic fermentation Hexose --- pyruvate --- propionate + acetate + CO2 + H2O Lactate --- pyruvate --- propionate + acetate + CO2 + H2O Propionibacterium Anaerobic Simple C-substances (mono-, disaccharides) Organic N-substances Aerotolerant Gastrointestinal tract (esp. rumen) Vitamin B12 production Cheese production - Swiss cheese (Emmenthal cheese), characteristic flavour and holes Regulation of fungi 9.1.2. Respiration Aerobic respiration of macromolecules Main phases: (1) hydrolysis –macromolecules breakdown (2) catabolic pathway – pyruvate formation (3) pyruvate decarboxylation and acetylKoA formation (4) tricarboxylic cycle (TCA) – use of acetyl-KoA in TCA – forming of citric acid and its biochemical changes; products: H2O, NADH, CO2, FADH2, ATP (5) respiration chain (electron transport chain, ETC) – oxidation of H+ and electron transport (NADH, FADH2) H+ + O2 + Pi + ADP H2O + ATP ETC is located in plasma membrane Several carriers participate in ETC (flavoproteins, cytochromes Result: C6H12O6 + 6O2 + 38Pi + 38ADP 6CO2 + 6H2O + 38ATP (=energy) The best sources of energy C-sources: cellulose, hemicellulose, starch, lignin; mono-, di- …. saccharides Organisms: several fungi and bacteria, very common process Main mineralisation process in Cturnover Aerobic respiration (non-complete) org C-subst. + O2 org C-subst. + (CO2) + H2O + energy (1) Acetic fermentation ethanol + O2 acetic acid + H2O+ E Energy = 6ATP Organism: Acetobacter Production of vinegar, spoilage of alcoholic beverages (wine, beer…) (2) Citric acid fermentation Saccharides+O2 citric acid + H2O + E (molasses) Organism: Aspergillus niger Citric acid production for food and beverage industry, pharmaceutical industry Anaerobic respiration In anaerobic condition oxygen (or other inorganic acceptor) from substances can be used for H+/e- oxidation Dissimilative denitrification: NO3- + H+ + Pi + ADP N2 + H2O + ATP Desulphurisation SO42- + H+ + Pi + ADP Methane formation CO2 + H+ + Pi + ADP S + H2O + ATP CH4 + H2O + ATP Assimilative denitrification: NO3- + H+ + Pi + ADP NH4+ +H2O+ ATP 9.2. Anabolism = Biosynthesis The use of energy and nutrients to construct cell constituents. Several enzymes from catabolism are shared. Basic schema: Inorganic molecules (CO2, NH4+, H2O, PO43-) (AUTOTROPHS) Monomers, Building blocks (nucleotides, sugars, amino acids, fatty acids) (HETEROTROPHS) Macromolecules (nucleic acids, proteins, lipids, polysaccharides) Supramolecular systems (membranes, enzyme complexes) „Organelles“ (nucleoid, nuclei, ribosome, flagella) Cell (bacteria, fungi, protozoa) Biosynthesis in Escherichia coli: Cell constituent Number of molecules ATP required DNA 1 60 000 RNA 15000 75 000 Polysaccharides 39000 65 000 Lipids 15 000 000 87 000 Proteins 1 200 000 2 120 000 Fixation of CO2 - The high need for energy – trapping light during photosynthesis, or derive energy from the oxidation of reduced inorganic electron donors. - Autotrophic fixation of CO2 is crucial, it provides the organic matter for heterotrophs. - Incorporation of CO2 – several types: Calvin cycle, pentose phosphate cycle… - Carboxysomes typical for thiobacilli, cyanobacteria, nitrifying bacteria – site for CO2 fixation. Synthesis of sugars Gluconeogenesis – close to glycolytic pathway, three steps are easily irreversible, starts with pyruvate synthesis. Result – glucose or fructose from which other sugars are produced. Phosphorus assimilation - Necessary in nucleic acids, ATP, NADP, proteins, phospholipids, etc. - Easily incorporated through formation of ATP: (1) photophosphorylation (2) oxidative phosphorylation (3) substrate phosphorylation - Preferable forms: H2PO4-, HPO42- From organic substances released by phosphatases Sulphur assimilation Sulphur is needed for synthesis of amino acids (methionine, cysteine) and several cofactors (coenzyme A, biotin…). Sources: SO42- through assimilatory sulphate reduction, amino acids, H2S Ammonium assimilation Relatively easily incorporated through amination: Pyruvate alanin α-ketoglutarate glutamate oxalacetate aspartate Other amino acids synthesis through transamination Nitrate assimilation Nitrogen in NO3- is too oxidized and it must be reduced through assimilatory nitrate reduction NO3NO2NH3 (NH4+) Nitrogen (N2) assimilation (fixation) Nitrogen fixation occurs in: (1) free-living bacteria (Azotobacter, Clostridium, Klebsiella, Methanococcus) (2) symbiotic fixation (Rhizobium….) (3) cyanobacteria (Nostoc, Anabaema…) Reduction N2 with enzyme nitrogenase: N2 NH NH2 NH3 NH4+ N2 + 8H+ + 8e- + 16ATP 2NH3 + H2 + 16ADP + 16Pi Protein synthesis - Main place = ribosome - Activity of ribosome: the use of energy (light, chemical), interaction of r-RNA, m-RNA and t-RNA. - Three steps: initiation, elongation, termination. 8.3. Regulation Substrate concentration Esp. very low concentration Ks = Michaelis constant (substrate concentration in which the speed of enzyme reaction is half of maximum) Enzyme concentration Speed of m-RNA transcription and speed of enzyme synthesis in ribosomes Allosteric regulation Some molecules act as effectors, modulators These molecules change the enzyme conformation (result = increasing or decreasing enzyme activity) Feedback effect End product stops the enzyme reaction in very beginning E1 S E2 M1 E3 M2 E4 M3 E5 M4 P Oxygen effect Some anaerobes stop their metabolism in the O2 presence (sometimes O2 toxic) Some facultative anaerobes – change of metabolism: in O2 presence respiration, in O2 absence fermentation (= Pasteur effect) Environment factors Minimum – optimum – maximum Temperature, pH…