biology midterm review – lutz 2013

BIOLOGY MIDTERM REVIEW – LUTZ 2013
THIS BELONGS TO: _______________________________
***KEEP IN MIND: THIS IS INTENDED FOR REMINDING YOU OF THINGS WE HAVE COVERED IN CLASS. IT IS NOT
INCLUSIVE. LOOK OVER YOUR NOTEBOOK (OLD WARM-UPS, WORKSHEETS, NOTES, VOCAB, TESTS, QUIZZES AND
LABS). ALL MATERIAL IS FAIR GAME FOR THE MIDTERM! For 40 points this review packet is due the day of the
Midterm, completely filled out (no exceptions)!
CHAPTER 1: WHAT IS SCIENCE? (pp 2-33)
What are the parts of the Scientific Method? Explain each.
-
-
-
-
-
Explain each of the following parts of an experiment:
Independent Variable
Dependent Variable
Constants
Control Group
Experimental Group
Define the following terms:
Hypothesis
Scientific Theory
Scientific Law
Homeostasis
List at least 5 Lab Safety Rules:
What are the 4 basic units of measurement in the Metric System (ex. Volume is ___________)
Be able to convert between metric units. (ex kg to mg)
Explain the 7 characteristics of living things we discussed in class (P 16).
-
-
-
-
-
-
-
Identify and define the 3 levels of organization we discussed in class (P 21):
-
-
-
CHAPTER 19: VIRUSES AND BACTERIA (pp 470-491)
How do we classify and Identify Prokaryotes?
Draw and Label a Bacteria (Prokaryotic cell).
DEFINE:
Virus
T4 Bacteriophage
Lytic Cycle
Lysogenic Cycle
Capsid
Retrovirus
CHAPTER 18: CLASSIFICATION (pp 446-465)
What are the 4 main reasons to classify living things?
How did Linnaeus group organisms in his classification system?
Which 2 Kingdoms did Linnaeus recognize?
What is binomial nomenclature? Give an example.
List the 8 levels of taxonomy (modern levels of classification). From LARGEST (least specific) to
SMALLEST (most specific)
-
-
-
-
-
-
-
-
Explain the modern ways of classifying (cladogram/molecular clocks)
How many Kingdoms are there? How many Domains?
What are the main characteristics of each of the Domain? The Kingdoms?
Be able to use a dichotomous key and cladogram.
List the characteristics of organisms in the Phylum Chordata.
ADDITIONAL VOCABULARY: taxonomy genus taxon evolutionary classification derived characteristics
CHAPTER 2: THE NATURE OF MATTER (pp 35-57)
What are the three subatomic particles of an atom? What are their characteristics? Where are they found in the atom?
What are isotopes?
What are ions?
What are elements?
What are compounds?
How are ionic and covalent bonds similar? How are they different?
What is a hydrogen bond?
What are solutions/suspensions?
Draw a molecule of water – H
2
O
Define the properties of water that make it a unique molecule. Give an example of each.
VOCAB:
Capillary Action
Cohesion
Adhesion
Surface Tension
Evaporation
Solid form is less dense than liquid form
Fill in the following chart:
Monomer Polymer Organic
Compound
Carbohydrate
Lipid
Protein
Nucleic Acid
What is pH?
What are acids? Bases?
What is a buffer?
What are enzymes?
List 3 things that can affect/regulate enzyme activity.
Function Example
ADDITIONAL VOCABULARY:
Atom
Atomic number
Atomic mass
Nucleus
Cohesion
Adhesion
Mixture
Solution
Solvent
Solute
Suspension
Reactant
Product
Catalyst
Substrate
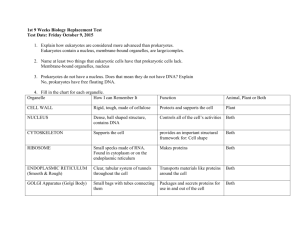
CHAPTER 7: CELL STRUCTURE AND FUNCTION (pp 168-197)
What are the 3 parts of the Modern Cell Theory?
What are the three parts of the Traditional cell theory?
Who were the scientists involved in the development of the cell theory? What was their contribution?
What are the four levels of organization in a living organism from smallest to largest? Define: tissue
What are the similarities and differences between prokaryotes and eukaryotes?
What are the similarities and differences between plant cells and animal cells?
What are the functions of the main organelles we studied?
VOCAB:
Cell membrane
Cell wall
Nucleus
Cytoplasm
Nucleolus
Ribosomes
Endoplasmic reticulum (rough and smooth)
Golgi apparatus
Lysososmes
Vacuoles
Chloroplasts
Mitochondria
Explain the 6 ways of getting things in/out of cells. What is unique about each one?
What are the different solutions (isotonic/hypertonic/hypotonic) and what happens to cells in those solutions?
CHAPTER 8: ENERGY AND LIFE (pp 200-219)
What are autotrophs? What are heterotrophs? Give an example of both.
What is ATP? How is energy stored in the cell? How is energy released in the cell?
What is the equation for photosynthesis?
Describe the 2 reactions of photosynthesis. Where does each process take place?
CHAPTER 9: ENERGY AND LIFE (pp 220-239)
What is the equation for cellular respiration?
Explain/write out the reactants and products of Lactic Acid Fermentation
Explain/write out the reactants and products of Alcoholic Fermentation
*** Cellular Respiration results in the production of 36 ATP molecules
Process
Glycolysis
Krebs or Citric
Acid Cycle
Electron
Transport Chain
Fermentation
Total
ATP release Where in the cell?
36
Need O2 Type of
Respiration
CHAPTER 12: DNA AND PROTEIN SYNTHESIS (pp 286-312)
Name the (groups of) scientists and their main accomplishments towards the study of DNA.
What is Chargaff’s Rule, and how does it work.
Draw and label a double helix structure.
Name the four nucleotides, label the primines and pyrimidines:
Why is a bacteriophage also called a transformer?
What are the three types of RNA and where can they be found.
Outline the basic steps of transcription and translation:
VOCABULARY
Anticodon
Codon
Exon
Intron
Polymerase
Histone
*** Only material from ch. 12 lectures prior to the midterm will be included in exam.