Supplementary Methods
advertisement

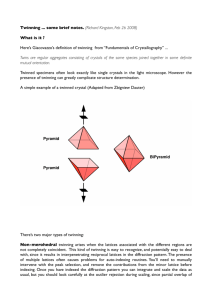
Supplementary Methods Twinning and pseudocentering in VP5CT crystals formed using MPD as a precipitant has been described in a separate publication1. In these crystals, 12 molecules of VP5CT pack in each asu of space group P4(2) with perfect hemihedral twinning. The manifestation of twinning in the intensity distribution of diffracted X-rays is obscured by the effects of pseudo I-centering, but can be demonstrated by a local intensity distribution statistic1 or by second moment analysis of only those acentric reflections with indices h+k+l=2n, where n is an integer (the strong reflection class). Because the difference between twin blocks is small (probably a rotational difference, although the twin operator has not been determined), the twinning is only statistically apparent (as indicated by I2/I2 < 2 for the strong reflection class, where I is intensity) beyond 8.3 Å resolution. Below 8.3 Å, the crystals approximate P4(2)22 with 6 molecules per asu. Due to the pseudocentering, below 12.8 Å resolution, the mean intensity of acentric reflections with h+k+l2n (the weak reflection class) is less than 2 of the intensity distribution of the strong reflection class. Thus, below 12.8 Å, the crystals approximate I422 with 3 molecules per asu. The twinning and non-isomorphism (based on different resolution limits of detectable twinning and pseudocentering in crystals with similar unit cell parameters) prevented structure determination with diffraction data from these crystals. To overcome this twinning disorder, we optimized conditions to obtain crystals with a sufficiently small rotational difference between twin blocks to allow an untwinned molecular model to adequately fit the diffraction data to a useful resolution. To identify such conditions, crystals were screened by second moment analysis of the strong reflection class from complete data sets. Reflection data were sorted into strong and weak classes using sftools2, and the second moment analysis of the acentric intensity distribution within classes was performed using truncate2,3. 1 Multiple wavelength anomalous dispersion (MAD) data for phasing were obtained at Advanced Photon Source beamline ID19, using inverse beam geometry at wavelengths of 0.97940 Å (selenium peak), 0.97954 Å (inflection), and 0.96148 Å (remote). All diffraction data were obtained at 100 K. The crystal of selenomethionine-substituted VP5CT used to obtain starting phases has refined unit cell lengths of a=b=243.3 Å and c=104.8 Å. This crystal shows no statistically apparent twinning to its diffraction limit of 3.5 Å. The intensities of the weak reflections become significant (as defined above) beyond 7.4 Å resolution. Data from this crystal were integrated and scaled in space groups I422 (for initial heavy atom searches) and P4(2)22 (for additional heavy atom searches, refinement, and phasing). After indexing and separate scaling using HKL 2000 (HKL Research, Inc.), data sets obtained at each wavelength for the MAD phase determination were scaled to each other using scaleit2,4 (Supplementary Table 4). Native data were obtained at CHESS beamlines A1 and F1. The native crystals obtained in the ammonium sulfate condition have refined unit cell lengths of a=b=244.8 Å and c=104.8 Å. Diffraction data from these crystals scale well to 2.7 Å resolution (R merge=0.38 and I/=3.7 in the highest resolution shell). The mean intensity of the weak class of reflections is less than 2 of the intensity distribution of the strong class below 7.1 Å resolution. Perfect hemihedral twinning is statistically apparent beyond 3.2 Å resolution. Data between 20 and 3.2 Å were scaled in space group P4(2)22 and used for structure determination. Reflections from two isomorphous crystals were scaled together using HKL2000 for the native data set (Supplementary Table 4). Initial selenium positions were located by direct methods, using SnB5, based on the anomalous signal at the selenium peak wavelength of the MAD dataset. Sites common to searches in spacegroups P4(2)22 and I422 were used to calculate initial anomalous difference maps. Based on difference maps calculated in P4(2)22 and on the ncs apparent in the distribution of the initial selenium positions, 30 seleniums were located in each asu. 2 Selenium positions were refined and starting phases calculated with mlphare,2,6 treating MAD as a special case of multiple isomorphous replacement with anomalous scattering. The inflection wavelength data set was designated “native” for calculating “isomorphous” differences (Supplementary Table 4). The figure of merit (FOM) for the experimental phases is 0.329. These weak experimental phases were substantially improved by solvent flipping (taking advantage of the 67% solvent content of the crystals) and histogram matching using dm2,7 to yield a density modified FOM of 0.649. After 6-fold maskless averaging using maprot2,8, the resulting electron density map, calculated with data between 15 and 3.5 Å, was skeletonized and the protein main chain was traced in o.9 The alignment of the primary sequence to the protein chain trace was guided by the selenium positions and the electron density of bulky side chains. The selenomethionine and native data sets were sufficiently isomorphous to allow transfer of phase information by rigid body refinement of the initial model to the native data set. Phase extension based on the 6-fold ncs was carried out from 3.5 to 3.2 Å using dm2,7. The model was improved through multiple cycles of manual rebuilding in o9 and simulated annealing refinement using 6-fold restraints in CNS10. Density modification using the ncs in dm2,7 and simulated annealing omit maps calculated in CNS10 were used to avoid model bias in the rebuilding. Masks for ncs averaging and solvent flipping were constructed using mama11 and o9. Symmetry operators were calculated using lsqman12. Model quality was analyzed using Procheck13. Solvent-accessible surface area was calculated using areaimol14. Cavities were modelled using cavenv2 and their volumes calculated using mama11. A sample of the final electron density in a simulated annealing omit map is given in Supplementary Fig. 2. References for Supplementary Methods 3 1. Padilla, J. E. & Yeates, T. O. A statistic for local intensity differences: robustness to anisotropy and pseudo-centering and utility for detecting twinning. Acta Crystallogr D Biol Crystallogr 59, 1124-1130 (2003). 2. Collaborative Computational Project, N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D50, 760-763 (1994). 3. French, G.S. & Wilson, K.S. On the treatment of negative intensity observations. Acta Crystallogr A Found Crystallogr A34, 517-523 (1978). 4. Howell, L., & Smith, D. Normal probability analysis. J Appl Cryst 25, 81-86 (1992). 5. Weeks, C. M. & Miller, R. The design and implementation of SnB v2.0. J Appl Cryst 32, 120-124 (1999). 6. Otwinowsky, Z. Maximum likelihood refinement of heavy atom parameters. CCP4 Daresbury Study Weekend Proceedings, 80-86 (1991). 7. Cowtan, K. D. 'dm':An automated procedure for phase improvement by density modification. Joint CCP4 and ESF-EACBM Newsletter on Protein Crystallography 31, 34-38 (1994). 8. Stein, P. E. et al. The crystal structure of pertussis toxin. Structure 2, 45-57 (1994). 9. Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for binding protein models in electron density maps and the location of errors in these models. Acta Crystallogr A 47, 110-119 (1991). 10. Brunger, A. T. et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54, 905921 (1998). 11. Kleywegt, G. J. & Jones, T. A. Software for handling macromolecular envelopes. Acta Crystallogr D52, 842-857 (1999). 4 12. Kleywegt, G. J. Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr D52, 842-857 (1996). 13. Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the sterochemical quality of protein structures. J Appl Cryst 26, 283291 (1993). 14. Lee, B. & Richards, F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol 55, 379-400 (1971). 5