Western
advertisement

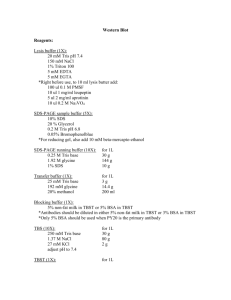
Western Blotting SDS-PAGE Protocol with BioRad Gel Assembly Gel protocol Prepare 1X Running Buffer (RB) from 5X solution (100 ml/run) Assemble gel apparatus with BioRad pre-cast gel (see manufacturer’s protocol) Cut sealing strip from bottom of pre-cast gels If using only one gel, use blank assembly for other side Electrophoresis Tank Running Buffer Gel Holding Tank Black Contact Blank, If Only One Gel Red Contact Gel with Wells to Inside Locking Tabs Pour 1 L of 1X RB into tank Remove comb from gel (carefully) Wash wells with RB Make sure no air bubbles in wells or below gel Use 5% or 10% Tris-Cl gels Combine 50 ug (or maximum possible) solubilized sample with 5X SDS-Loading Buffer Heat to 85° C for 5 minutes Well capacity is ~ 50 uL Load 10 uL Kaleidoscope Standard Proteins + 2 uL 5X SDS-Loading Buffer Eluted samples don not have to be heated to 85 C again Optional: Add 10 uL diluted, heat denatured Biotinylated Standards to well, with 5X SDS-Loading Buffer. May contribute to background Run at 150 V for 45 minutes at RT Make sure wells remain submerged below the RB Do not run standard beyond gel bottom Prepare 1X Transfer Buffer (TB) Use cold qH2O Methanol shrinks the membrane pore size Supplement TB with 1% Tween-20 Facilitates transfer of proteins larger than 100kDa Prepare PVDF membranes, cut to size of gel (simultaneously with next step) Pre-wet membranes in 100% methanol, then incubate in TB for 10 minutes Trim one corner to mark protein side Use TBST for PVDF membranes (smaller proteins) Trim gel and prepare “sandwich” Cut 0.5 cm off bottom and all wells off top Soak gel, blotting papers and filter pads in TB for 10 minutes Assemble sandwich in TB, rolling and wetting each set Do not let membranes go dry Assemble as shown below: White Side Fiber Pad Blotting Paper Membrane Gel Blotting Paper Fiber Pad Black Side Place “sandwich” in tank, along with ice block Transfer Tank Transfer Buffer Ice Block Red Contact Stirrer Black Side White Side Blotting Apparatus Holder Black Contact Blotting “Sandwich” Apparatus Locking Tab Transfer at 40 V overnight or 60 V for 3 hours Perform in cold room, while stirring constantly Protein standards should be completely transferred from gel to protein when done Rinse membrane with TBST Block non-specific interactions with 5% milk in TBST Supplement with protease inhibitors Incubate overnight at 4° C or 1 hour at room temperature Place membrane in pipette tip box lid. Cover with aluminum foil and label Milk blocking solution should be freshly prepared Tween-20 helps reduce background Membrane is viable for 1 week if stored in 1X TBST at 4° C Continue as follows… Blotting Protocol Wash membrane 2X quick, 3X-15 minutes, 2X-5 minutes; all with shaking in TBST Use largest volume of wash buffer possible Add primary antibody at appropriate dilution, incubating 2-3 hours with shaking Anti-GFP Ab – diluted 1:1000 in 1X TBST Do not let membrane go dry after incubation Save primary antibody solution for re-use Wash membrane 2X quick, 3X-15 minutes, 2X-5 minutes; all with shaking in TBST Add secondary antibody, 1:2000 for 1 hour 7.5 uL secondary Ab/15 ml 1X TBST If RIM2 Ab, dilute 1:500 in 1X TBST To save on antibody, use smaller volume Place membrane in plastic weighing boat, add just enough volume to cover Wash membrane 2X quick, 3X-15 minutes, 2X-5 minutes; all with shaking in TBST Add tertiary antibody, 1:2500 for 1 hour 6 uL tertiary Ab/15 ml 1X TBST Wash membrane 2X quick, 3X-15 minutes, 2X-5 minutes; all with shaking in TBST ECL Detection Wear powder free gloves Place blot flat on Saran wrap with protein side up Blot corner of membrane to remove excess buffer Pipet ECL reagents (1 Luminol:1 H2O2 (v/v) onto protein side of membrane Use just enough reagent to cover membrane Incubate exactly 1 minute, without agitation Work quickly from this pont Drain membrane, blotting corner to remove excess reagent Cover membrane completely with Saran wrap Trim excess Smooth away air pockets Develop image (film, etc.) in darkroom Apply protein side of membrane against ECL film Incubate as needed Stripping Membranes Incubate membrane in Strip Buffer (SB) for 30 minutes at 70° C Wash 1X with TBST Re-blot for second protein as in steps 12-20 above Reagents 5X Running Buffer (RB) 15 g Tris Base 72g Glycine 5g SDS To 1 Liter H2O Do not pH (8-9) Stripping Buffer (SB) 750 uL B-mercaptoethanol 2g SDS To 100 ml 1X TBS 10X Transfer Buffer (TB) 58 g Tris Base 29 g Glycine To 1 Liter H2O 10X TBS 200 ml Tris Base 80 g NaCl To 1 Liter H2O 1X TBST 1 ml Tween-20 999 ml 1X TBS To 1 Liter H2O Do not pH (8-9) pH to 7.6 Mix Well Trituration Buffer 2% Triton X-100 10 mM Tris Base 1 mM EDTA Protease inhibitors Mix Well Lysis Buffer 0.5 % SDS 0.05M Tris-Cl 1 mM DTT Protease inhibitors pH to 8.0 TBST+ 5% Milk 2.5 g Dry Milk To 50 ml 1X TBST Protease Inhibitors Name PMSF(Phenylmethanesulfonyl Fluoride) Aprotonin Leupeptin Pepstatin Benzmidine Pefabloc SC Calpain Inhib. I+II [Working] 1.5 mM 10 µg/ml 10 µg/ml 10 µg/ml 1 mM 0.4 mM 8 µg/ml V for 10ml Buffer 30 µL 6.7 µL 20 µL 10 µL 10 µL 10 µL 8 µL [Stock] 0.5 M 1.5 mg/ml 5 mg/ml 10 mg/ml 1M 400 mM 10 µg/µL Stock Storage -20° C 4° C -20° C -20° C -20° C -20° C -20° C Storage Media H20 H20 H20 H20 H20 H20 (EtOH)