Western Blotting using the BioRad Criterion System

(Carrie Mosher, 2009)
Western Blotting using the BioRad Criterion System
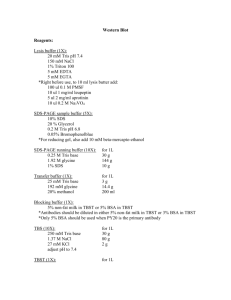
Note: For the Criterion system, you can run a single gel without the need for a buffer dam
1.
Place gel in the electrophoresis box, with the attached “bucket” facing inward
Be sure to remove white strip at bottom of gel
2. Fill box with 1X Tris-Glycine-SDS Running Buffer to “Fill” line
3. Fill “bucket” with same buffer until top
4. Dilute samples with 4X Laemmli Sample Buffer
Use PCR tubes, either strips or singles
5. Boil samples in the PCR machine for 5 min at ~100°C
e.g. program 57 on “Snow Leopard”
6.
In centrifuge, do a “short spin” to recollect samples on bottom of tubes
7. Using a Hamilton syringe with a flat tip, add samples to each well
18-well gels hold up to 30
l per well
Add 20-25
l samples, 10
l protein ladder
8. Surround gel box in red tub with ice to prevent overheating and place lid on (match redred, black-black)
9. Set power source to constant Amps, and run at 0.06 A
If using gradient gel, amps will fall below 0.06 A as voltage max is reached
Make sure see bubbles in both chambers
Run ~1 hr until dye band runs off bottom of gel
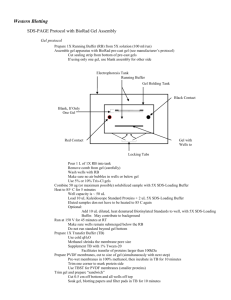
10. When gel is close to finished running, prepare the blotting membrane
11. Cut PVDF membrane to size of gel (~13 × 5.5 cm)
Before touching membrane, rinse gloved hands with DI water
Membrane needs to fit in as small a container as possible
Cut top left corner to mark it
12. Soak membrane in methanol for ~1 min
13. Dump methanol, add 1X Transfer Buffer containing 10% methanol
Rock for ~5 min, until can see the membrane sink and is no longer beading water
Also soak 2 blotting papers in a separate container
14. When gel is done, crack open cassette
(Carrie Mosher, 2009)
use spatula and carefully crack all the way around before opening
15. Let gel fall onto side of cassette that does not have the “bucket”
16. Using edge of glass plate or razor blade, cut gel down to size of membrane
Also cut top corner to mark where lane 1 is (is now on right hand side)
17. Place membrane on top of gel so marked corners match
Only place membrane on gel once or can get “ghost” bands on blot
18. Place moist blotting paper over the membrane
Carefully flip “sandwich” onto the bottom plate of the semi-dry transfer cell
19. Carefully peel off gel cassette
20. Add a little Transfer Buffer on gel and roll with Falcon tube to remove air
21. Add top blotting paper, roll again
22. Add top plate and lid of transfer cell
23. Set power source to constant Voltage, run at 25 V for 30 min
24. While is running, prepare milk for blocking
Make 10% dry milk solution in 1X TBST (TBS Buffer + Tween)
5 g milk in 50 ml Falcon tube
Make sure milk is fully dissolved, no chunks
25. After 30 min, remove lid, then carefully remove top plate
Sandwich may attach to top plate
26. Carefully peel back gel to ensure complete transfer
Bands of protein ladder should be visible on membrane not on gel
27. Wash membrane for 2-3 min in TBST with fast rocking
28.
Block membrane overnight in 10% milk solution with slow rocking at 4°C
Add ~40 ml of milk to fully cover membrane
Cover with plastic wrap or aluminum foil
Put remaining milk in refrigerator for use the next day
29. The next day, warm membrane up to room temperature
30.
Wash 3× (every 5 min) with TBST and fast rocking
31. Incubate (with slow rocking) for 1 hr with primary antibody in 1% milk solution
For UGT1A6 185-2 antibody, dilute 1:500
Use 1.5 ml of 10% milk + 30
l Ab
in 15 ml Flacon tube, fill with TBST
(Carrie Mosher, 2009)
32.
Wash 3× (every 5 min) with TBST and fast rocking
33. Incubate for 1 hr with secondary antibody
For UGT1A6 Gentest goat anti-rabbit HRP-conjugated antibody 1:500 dilution
34.
Wash 3× (every 5 min) with TBST and fast rocking
35. Add chemiluminescent substrate
Mix 1 ml of each solution together
Pipette slowly over membrane
36. Incubate for 5 min
37. Use Kodak Image Station to illuminate
Place membrane and a little liquid on imager
In software, check “preview” box and then click “expose” to position membrane properly (may need to zoom camera in)
When it is the way you want it, click “stop” and uncheck “preview”
38. Close lid securely
39. Collect data for 16 min with 30 s capture intervals
40.
When complete, click “submit” and “enter”
Can then save image, quantify, or export as image file