Western blot
advertisement

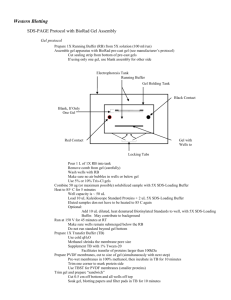
MCB730 Western blotting First Lab Meeting Protein extraction 1. Filter the cell culture (GFP fusion protein expressing tobacco BY-2) to separate cells from culture medium. Use the cells for protein extraction and western blotting and the medium for dot blotting. 2. Add 2-mercaptoethanol to 2X SDS-PAGE loading buffer to a final concentration of 10%. 3. Grind ~100mg cultured cells in liquid nitrogen. 4. Add the fine powder into an equal volume of 2X SDS-PAGE loading buffer (100l) and mix by vortexing. 5. Denature the sample at 100 ºC for 5 minutes. 6. Centrifuge for 10 min at maximum speed in a bench top centrifuge at room temperature. Transfer supernatant to a clean tube. Western blotting (Electrophoresis, transfer and blocking) 1. Set up the gel electrophoresis apparatus with Biorad Ready Gels. 2. Load each well with 25l proteins from above. The TA will load protein markers. Load two identical gels. 3. Run the gel at 200 volts in 1X Tris-glycine-SDS electrophoresis buffer. 4. When the dye arrives at the end of the gel, stop electrophoresis. 5. Stain one gel with 0.25% Coomassie Brilliant Blue R-250 for 30 min at RT. 6. Destain the gel in destaining buffer until the bands are clearly visible. 7. Use the other gel for western blotting. 8. Rinse the gel in the transfer buffer for 15 min. In the mean time, cut PVDF and filter paper to the exact dimensions of the gel. 9. Cut one corner of the PVDF to mark orientation. Wet it briefly in 100% methanol and then submerge it in the transfer buffer, along with 2 pieces of filter paper. 10. Set up the electrotransfer apparatus according to the following order: Cathode(-)-pad-filter-gel-PVDF-filter-pad-anode(+) 11. Transfer at 100V for 1 hour. 12. Wash the membrane in ddH2O. 13. Proceed directly to blocking or air dry the membrane for later use, if desired. 14. Block the membrane in blocking buffer at 4 ºC until the next lab. Dot Blotting 1. Add 1l of the culture medium and protein sample to the nitrocellulose membrane and allow these spots (i.e., dots) to dry completely. 2. Block and blot same as the PVDF membrane in western blotting. Second Lab Meeting Immuno-blot and colormetric development 1. Dilute the GFP antibody 1:400 with blocking buffer (25l into 10ml). Incubate the membranes for 1 h at RT with gentle agitation. 2. Wash the membrane 3 times with TTBS (TBS containing Tween-20), 5 min each time with gentle agitation. 3. Dilute alkaline phosphatase-conjugated secondary antibody 1:3000 with blocking buffer (3.3l into 10ml). Incubate the membrane for 1 h at RT with gentle agitation. 4. Rinse the membrane 3 times with TTBS, 5 min each time. Rinse the last time in TBS without Tween-20. 5. Prepare the color substrate according to the manufacturer’s instructions. Develop color in the substrate solution for a few minutes. Do not shake. 6. Stop color development by immersing the membrane in water. 7. Dry the membrane and store away from light. Recipes 2X Laemmli SDS-PAGE loading buffer 100mM Tris, 4% (w/v) SDS, 0.2% (w/v) bromophenol blue, 20% (v/v) glycerol, pH 6.8 Add 2-mercapoethanol to a final concentration of 10% right before use. Tris-glycine-SDS electrophoresis buffer 25mM Tris, 250mM glycine, 0.1% SDS, pH 8.3 Transfer buffer 25mM Tris, 192mM glycine, pH 8.3 TBS 20mM Tris, 500mM NaCl, pH 7.5 TTBS TBS, 0.05% Tween-20, pH 7.5 Blocking buffer 5% nonfat milk powder in 1X TBS Coomassie blue staining buffer 0.25% (w/v) Coomassie blue R-250, 50% methanol, 10% glacial acetic acid, 40% water Destaining buffer 50% methanol, 10% glacial acetic acid, 40% water