Experimental Protocol for H2AX Western Blotting
advertisement

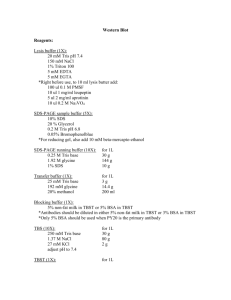
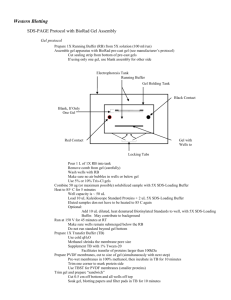
Molecular and Clinical Radiobiology Workshop Lab Practical Tutorial Sessions RI-MUHC June 19th, 2015 PROTOCOL Analysis of H2AX phosphorylation by Western Blot Cell culture and treatment Culture the cells on 100mm cell culture dishes, according to the requirement (modified Eagle’s MEM supplemented with 10% fetal bovine serum (FBS), vitamins, sodium pyruvate, L-glutamine, penicillin, streptomycin, and nonessential amino acids at 37°C in the presence of 5% CO2). Treat the cells as necessary with radiation (e.g., 2-10 Gy) Incubate the cells in a CO2 incubator at 37 °C for 0 min, 5min, 30min, 60min, 6hs and 24hs. Protein extraction and quantification Wash cells with cold PBS and then lyse with ice cold RIPA buffer for 45 minutes at 4°C. Centrifuge the extracts at 12000g for 15minutes at 4°C. Collect the supernatant containing total extracted proteins. BCA protein assay kit (Pierce Scientific Ltd) will be used for protein quantification. SDS-PAGE and Western blot Load samples (30-50ug protein) on a polyacrylamid gel (4.0% stacking gel and 15% running gel). Run gel at 100 V (Bio-Rad Mini-Protean gel electrophoresis system will be used). Transfer proteins from the gel to the membrane Equilibrate gel in transfer buffer for 15 minutes. During equilibration, cut nitrocellulose to the size of the gel; wet in water and soak in transfer buffer. For each gel, cut 2 pieces of 3 MM paper to size of gel and soak in transfer buffer. Wet pads with transfer buffer. Assemble transfer unit as outlined below: (Red) pos. pole> clear plate> pad> 3 MM> nitrocellulose> gel> 3 MM> pad> black plate> neg. pole (Black). Make sure no bubbles are trapped Close the sandwich board and dunk into partially filled transfer chamber. 1 Molecular and Clinical Radiobiology Workshop Lab Practical Tutorial Sessions RI-MUHC June 19th, 2015 Put in "BioIce" and fill chamber to top, but do not over fill. Run transfer at 30 volts overnight in a cold room . Verify transfer by staining with Ponceau S dye Block membrane for 1 h at room temperature on an orbital shaker using 5% (w/v) non fat milk in TBST. Incubate with primary antibody diluted in TBST (1:1000 - Phospho-Histone H2A.X (Ser139) (20E3) Rabbit mAb #9718 Cell Signaling) overnight at 4 °C on an orbital shaker. Pour off primary antibody solution and wash 3 x 10 min in TBST at room temperature. Incubate with secondary antibody (Anti-rabbit IgG, HRP-linked Antibody #7074 - Cell Signalling) diluted (1:2,000) in 5% milk in TBST for 1 hr at room temperature on an orbital shaker. Pour off secondary antibody solution and wash 3 x 10 min in TBST at room temperature. ECL chemi-luminescence [Amersham Biosciences (GE)] will be used to detect protein bands. Manual band quantification will be carried out using Spectrum Image and GelQuant® software. Reagents Lysis buffer (RIPA) 10 mM Tris-Cl (pH 8.0) 1 mM EDTA 0.5 mM EGTA 1% Triton X-100 0.1% sodium deoxycholate 0.1% SDS 140 mM NaCl 1 mM PMSF Add Protease and Phosphatase inhibitors (Roche 04693132001 and 04906845001) Protein sample buffer 40 mM Tris-HCl, pH 6.8 8 M Urea 5% (w/v) SDS 0.1 mM EDTA 1% (v/v) β-Mercaptoethanol (freshly added) 0.1 g/L Bromphenolblue Running buffer 25 mM Tris 192 mM Glycine 0.1% (w/v) SDS 2 Molecular and Clinical Radiobiology Workshop Lab Practical Tutorial Sessions RI-MUHC Transfer buffer 25 mM Tris 192 mM Glycine 20% (v/v) Methanol Check the pH and adjust to pH 8.3 if necessary Ponsceau S - store at RT 0.1% w/v Ponsceau S in 1% v/v acetic acid Tris-buffered saline (TBS ) 25 mM Tris 140 mM NaCl 2.5 mM KCl pH 7.4 Tris-buffered saline with Tween 20 (TBST) TBS with 0.1% (v/v) Tween 20 Phosphate buffered saline (PBS) 140 mM NaCl 2.5 mM KCl 8.1 mM Na2HPO4 1.5 mM KH2PO4 pH 7.3 3 June 19th, 2015