Western Blotting
advertisement

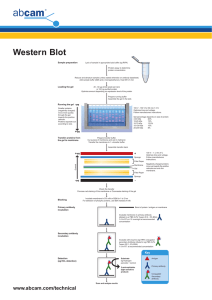
Semi-dry protein transfer, Western Blotting and Imaging Materials for Semi-dry protein transfer: Chromatography paper Grade 3, Catalogue number: 3030-861 Whatman Nitrocellulose membrane, Catalogue number: 162-0116 Bio-Rad Laboratories Transfer buffer: TBS-T Tris, mw 121.14 NaCl, mw 58.44 Make 10xTBS (0.5M Tris, 1.5M NaCl, pH 7.5) TBS-T (1x TBS, 0.1% Tween20) Sandwich assembly and protein transfer: Wear gloves to avoid contamination Cut 4 gel sized filter paper sheets and one nitrocellulose membrane per gel. Incubate the gel in Transfer buffer for at least 15 min. Soak the filter papers and membrane in Transfer buffer Place two of the filter papers first on a clean surface of the negative electrode followed by the membrane, the gel and the two remaining filter papers. Roll a plastic pipette tip over to take away air bubbles (for each step). Absorb any extra buffer in tissue paper before putting the positive electrode on top of the sandwich and applying current. Run the protein transfer at 150mA for 42 min for one gel, or 61 min for two gels. You may wish to assess the efficiency of protein transfer with Ponceau staining (see Ponceau staining protocol). Western blotting: Materials: Blocking buffer: 3% w/v BSA in TBS-T (1.5g BSA in 50 ml TBS-T) Antibody as an example: HRV conjugated His-tag Mab (R&D MAB050H) NB: If the primary antibody is conjugated with HRP no secondary antibody is needed. Protocol for Western blotting: Once the semi-dry protein transfer is completed, insert the membrane into a 50 ml Falcon tube with 20 ml Blocking buffer for overnight incubation at 4°C on a roller mixer to prevent any nonspecific binding of antibodies to the membrane. Dilute your antibody in TBS-T as recommended in the user’s guide and incubate with the membrane for 1 hour at room temperature on a roller mixer. In the case of HRV conjugated Primary antibody for His-tag, the Mab is diluted 1:5000 in Blocking buffer. Wash the membrane with 4 x 25 ml TBS-T for 10 min at room temperature on a roller mixer and proceed to Imaging. Imaging: Materials: Imaging in the G-box using GeneSnap program at Astbury level 6 Material to take to level 6: Two gel sized transparent plastic sheets A receptacle Pipette and pipette tips Tissue paper Spray bottle of water Super Signal West Pico Chemiluminescent Substrate (Catalogue number 34087, Pierce) Instructions how to use the program should be found by the equipment, if not so, ask for assistance until you are confident to use the program. West Pico treatment of the membrane should be done immediately before the Imaging. Imaging protocol: Place the membrane on top of one of the plastic sheets. Mix in a universal the two solutions (of the Super Signal WestPico kit) at 1:1. Usually for one membrane it is 1ml from each. Incubate the membrane for 5 minutes. Decant the excess on a tissue and put the other plastic sheet on top of the membrane. Follow the G-Box protocol to detect chemiluminescent.