X, Y, Z, ZZ - Oregon State University
advertisement

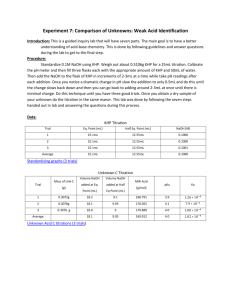
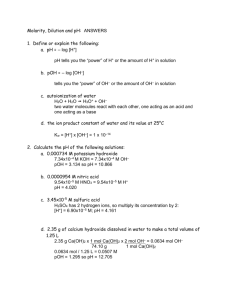
Chemistry 224H Week 3 Solutions October 4, 2006 Fall 2006 Oregon State University Dr. Richard Nafshun Problem X: Consider the balanced reaction SnCl2 (aq) + 2 FeCl3 (aq) → SnCl4 (aq) + 2 FeCl2 (aq) Write the two half-reactions. Identify the oxidation numbers of each species. Identify the reducing agent, the oxidizing agent, which species is oxidized, which species is reduced, and how many electrons are transferred. I will break down the overall reaction into two half-reactions: Fe3+ (aq) → Fe2+ (aq) Sn2+ (aq) → Sn4+ (aq) How did I determine the oxidation numbers? Why did I leave chloride ion out of the halfreactions? Fe3+ (aq) is being reduced to Fe2+ (aq). Recognizing the oxidation number decreased from 3+ to 2+ shows reduction: Here is the "iron" half-reaction showing the electron transferred: Fe3+ (aq) + e- → Fe2+ (aq) Sn2+ (aq) is being oxidized to Sn4+ (aq). Recognizing the oxidation number increased from 2+ to 4+ shows oxidation: Here is the "tin" half-reaction showing the electron transferred: Sn2+ (aq) → Sn4+ (aq) + 2 e- Summary: Fe3+ (aq) has gained an electron. Fe3+ (aq) is reduced. Fe3+ (aq) is the oxidizing agent. Sn2+ (aq) has lost two electrons. Sn2+ (aq) is oxidized. Sn2+ (aq) is the reducing agent. Problem Y: (a) A student places 20.53 grams of magnesium chloride into a 500-mL volumetric flask and fills to the mark with water. What is the concentration (molarity) of the solution? Does the molarity of the solution change if the solution is heated? 1 mol 20.53 g 95.21 g mol = 0.4313 M M= = 0.500 L L Molarity is temperature dependent and changes as temperature changes because the solution changes volume (L) as the temperature changes. (b) A student places 20.53 grams of magnesium chloride into 500-mL of water at 4 ºC. What is the concentration (molality) of the solution? Does the molality of the solution change if the solution is heated? 1 mol 20.53 g 95.21 g mol M= = = 0.4313 m 0.500 kg L Molality is not temperature dependent and does not change as temperature changes because the solution changes volume (L) as the temperature changes but not mass. Problem Z: A student titrates 0.6831 grams of KHP (potassium hydrogen phthalate, C6H4(COOK)(COOH), MW=204.2 g/mol) to the equivalence point with 35.42 mL of NaOH (aq). Determine the concentration of the NaOH solution. NaOH (aq) + KHP (aq) → NaKP (aq) + H2O (l) When the moles of NaOH added equals the moles of KHP (aq) in the sample, the flask contains salt and water. No KHP (aq) remains and no excess NaOH (aq) was added. The reaction is complete. At this point (known as the equivalence point): molesNaOH (aq) = molesKHP (s) Because KHP is weighed out (mass and molar mass were provided) leave the right side of the equation to enter moles. MNaOHVNaOH = molesKHP (s) (MNaOH) (0.03542 L) = 0.6831 grams of KHP 204.2 g/mol MNaOH = 0.09445 M or 0.09445 mol/L Problem ZZ: A student titrates 25.00 mL of HCl (aq) with 35.22 mL of 0.09227 M NaOH (aq) to reach the equivalence point. Determine the concentration of HCl (aq). molesNaOH (aq) = molesHCl (aq) MNaOHVNaOH = MHClVHCl (0.09227 M) (0.03522 L) = (MHCl) (0.02500 L) MHCl = 0.1300 M or 0.1300 mol/L