Comparison of Unknowns: Weak Acid Identification
advertisement

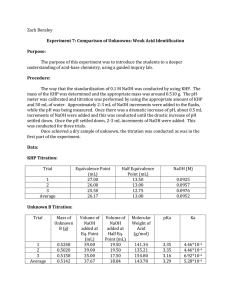
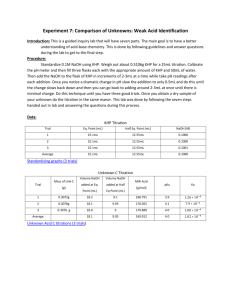
Experiment 7: Comparison of Unknowns: Weak Acid Identification Objective: To introduce you to a deeper understanding of acid-base chemistry using a guided inquiry lab. Procedure: 1. An unknown weak acid was obtained in order to be analyzed. 2. Three samples of KHP were dissolved in 50 mL of water. 3. The pH meter was calibrated and then the samples of KHP were titrated with a NaOH solution. 4. Then, pH readings were taken throughout the titration and a graph was formed. 5. The equivalence point and half equivalence points were determined on the graphs for each trial. 6. The weak acid was then titrated and pH readings were taken throughout in order to form a graph. 7. The molecular weight and Ka of the unknown acid were determined. Data: comparison of unknowns.xlsx Calculations: Grams of KHP To Produce 30mL of Titrant: Moles = 30mL × Molarity of NaOH 30mL × 0.10M = 0.003moles 204.23g 1molKHP mol molesNaOH × × 1mol NaOH 1mol KHP 1molKHP 204.23g/mol 0.003moles NaOH × × = 0.6127 grams KHP 1mol NaOH 1mol KHP Molarity of NaOH: Moles of Acid = Moles of Base L NaOH @ equiv. pt 0.003moles = 0.094M 0.032L pH Calculations: Before Base is Added moles KHP = M of HP − mL of water 0.00299mol KHP = 0.0598M HP − 0.05L HP − ↔ H + + P −2 I 0.0598 - C -x +x +x E 0.0598-x x x ka = - [H + ][P −2 ] HP − x2 = 3.98 × 10−6 = k a 0.0598 − x x = 4.88 × 10−4 − log(4.88 × 10−4 ) = 3.31 = pH At the Equivalence Point moles KHP = M of HP − mL water + mL NaOH @ equiv. pt 0.00299mol KHP = 0.0383M HP − 0.078L P 2− + H2 ↔ OH − + HP − I 0.0383 - C -x +x +x E 0.0383-x x x kb = - [HP − ][OH − ] [P −2 ][H2 ] x2 = 2.51 × 10−9 = k b 0.0383 − x x = 9.80 × 10−6 − log(9.80 × 10−6 ) = 5.01 = pOH pH = 14 − 5.01 = 8.99 Beyond Equivalence Point excess NaOH = [OH + ] 50 mL + 30mL + 1mL 2.8 × 10−4 = 0.00346M 0.081L − log(0.00346) = 2.46 = pOH 14 − 2.46 = 11.54 = pH How is a weak acid not completely dissociating taken into account when calculating pH and concentration? Because the acid does not completely dissociate, you have to calculate the H + concentration by factoring in an x. Therefore, you would then calculate the pH by taking the negative log of the value of x. Prediction of Curve The curve would have a small jump that is slightly steep. Two points on the curve help identify an unknown acid. These two points are the equivalence point, which is when the pH=7.0, and the half equivalence point, which is when half of the acid is consumed in the titration. Molar Mass of Unknown: (M NaOH)(mL of base at equivalence point) = moles base = moles acid (0.11M)(0.031L) = 0.00341mol acid and base 0.6126g of unknown acid × 1 = 179.651g/mol 0.00431mol pH at half equivalence point = pK a = 3.48 K a = 10−3.5 = 3.18 x 10−4 Conclusion: After performing my calculations, I determined that the unknown acid was acetylsalicylic acid. The calculated molecular mass from the experiment was 179.651g/mol. The molar mass of acetylsalicylic acid 180.16g/mol, which is very close to my experimental value. The literature value of Ka for acetylsalicylic acid is 3.2 x 10-4. My calculated Ka was 3.18 x 10-4, with a pH of about 3.5. These two numbers are extremely similar as well. In conclusion, this experiment had little error because of how close these two values were to the accepted values in the book.