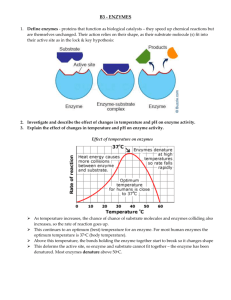
III. Properties of enzymes
advertisement

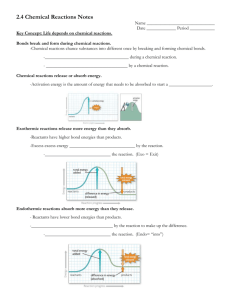
chpt 4. Enzymes II. Nomenclature A. Recommended name substrate+-ase ex. glucosidase (グルコシドを加水分解), urease(尿素を加水分解) etc. action+-ase ex. lactate dehydrogenase (ピルビン酸と乳酸の間の可逆変化を触媒), adenylate cyclase (ATP から cyclic AMP を生成する反応を触媒) etc. B. Systematic name by IUBMB chemical reaction+-ase 1. oxidoreductases 酸化還元酵素 2. transferase 転移酵素 transfers C, N or P containing groups 3. hydrolase 加水分解酵素 4. lyase 二重結合形成反応などを触媒する酵素の総称;cleaves C-C C-S C-N ex. decarboxylase 5. isomerase racemization of optical or geometric isomers racemization;ラセミ化,失旋:光学活性物質が右旋体と左旋体の等量混合物になり,光学的に不活性となること 6. ligase 連結酵素、合成酵素; ATP の加水分解を伴う共有結合を形成することによって、2 分子の結合を触媒する酵素の総称 III. Properties of enzymes increase the velocity of a chemical reaction not consumed during the reaction they catalyze A. Active site substrate-binding-site: a three-dimensional surface of amino acid side chains complementary to the substrate E+S -> ES -> EP -> E+P (p.52) B. Catalytic efficiency from 10^3^ to 10^8^ times faster than uncatalyzed reactions typically 100 to 1,000 substrate molecules into product per second turnover number: the number of molecules per enzyme molecule per second C. Specificity interacting with one or a few specific substrate, catalyzing only one type of chemical reaction D. Cofactors nonprotein cofactor: needed for enzymic activity ex. metal ions (Zn2+, Fe2+), coenzyme (organic molecules; NAD+, FAD, CoA) apoenzyme + cofactor -> holoenzyme cf. prosthetic group: coenzyme tightly bound to the enzyme (biotin of carboxylases) biotin: vitamin H; C10H16O3N2S:ビタミン B 複合体の一つで、結晶状、水溶性;生物の細胞 中に存在する成長因子 E. Regulation : can be activated or inhibited F. Location within the cell specific organelles within the cell 1. to isolate the substrate/product from other competing reactions-> regulation of the reaction 2. to provide a favorable environment 3. to organize the thousands of enzymes in the cell into purposeful pathways 1/5 chpt 4. Enzymes IV. How enzymes work Viewpoint energy changes during the reaction; enzymes provide energetically favorable reaction pathway how the active site chemically facilitate catalysis A. Energy changes occurring during the reaction free energy of activation: an energy barrier separating the reactants and the products energy difference between that of the reactants and a high-energy intermediate that occurs during the formation of product Rate of reaction: for molecules to react, they must contain sufficient energy to overcome the energy barrier of the transition state. In general, the lower the free energy of activation, the more molecules have sufficient energy to pass over the transition state, and thus the faster the rate of the reaction. Roll of enzymes: provide an alternate pathway with a lower free energy of activation. not change the free energies of the reactants or products and, therefore, does not change the equilibrium of the reaction. B. Chemistry of the active site active site: not a passive receptacle for binding the substrate promote the conversion of substrate to product 1. Transition-state stabilization: a flexible molecular template that binds the substrate in a geometry structurally resembling the activated transition state of the molecule (Cell, p.169 Fig 3-52) 2. Other factors: provide catalytic groups that enhance the probability that the transition state forms. general acid-base catalysis:一般酸塩基触媒作用; 酸のプロトンが部分的に移動したり、塩基がプロトンを部分 的に引き抜くことにより、transition state の free energy を低下させること (Voet p.199-) the transient formation of a covalent enzyme-substrate complex V. Factors affecting reaction velocity substrate concentration/temperature/pH A. Substrate concentration velocity: the number of substrate molecules converted to product per unit time -> µ (moles per minute) the more the substrate concentration, the more increases the velocity until maximal velocity(Vmax) level-off: the saturation with substrate of all available binding site on the enzyme enzymes following Michaelis-Menten kinetics: hyperbolic like myoglobin allosteric enzymes: sigmoidal like hemoglobin B. Temperature increase of velocity: increase of molecules having sufficient energy to pass over the energy barrier decrease of velocity: denaturation of the enzyme C. pH effect of proton on the velocity of catalysis catalytic activity may require that an amino group of the enzyme be –NH3+ at high pH this group is deprotonated => the rate of reaction declines extremes of pH can denature enzymes; changing the ionic character of the amino acid side chains => result in the change of the conformation pH optimum: ex. pepsin pH2 trypsin pH6 2/5 chpt 4. Enzymes VI. Michaelis-Menten equation (Voet chpt.12) B. Assumptions; 1. [S]>[E] 2. [ES] : constant: V1= V-1+V2 3. Initial velocity is solely used in the analysis : the rate of the back reaction from P to S can be ignored C. Conclusions 1. Km=[S] (when V=1/2Vmax, enzymes are half-saturated) small Km (= small [S]): high affinity of the enzyme for substrate large Km (= large [S]): low affinity of the enzyme for substrate 2. Velocity and enzyme concentration the rate of the reaction is proportional to the enzyme concentration 3. Order of reaction first order : velocity is proportional to [S] (when [S] << Km) zero order : velocity is independent of [S] (when [S] >> Km) D. Lineweaver-Burke plot (Voet p.225) it is not always possible to determine when Vmax has been achieved, because of the gradual upward slope of the hyperbolic curve at high substrate concentration => double reciprocal plot X-intercept is -Km^-1^ Y-intercept is Vmax^-1^ VII. Inhibition of enzyme activity reversible inhibitor: through noncovalent bonds irreversible inhibitor: sometimes through covalent bonds with specific groups of enzymes ex. certain insecticides form covalent bonds with acetylcholinesterase acetylcholinesterase: acetylcoline を choline と酢酸に加水分解して不活性化する酵素 A. Competitive inhibition the inhibitor binds reversibly to the same site that the substrate would normally occupy the effect is reversed by increasing [S] : Vmax is the same increases the apparent Km for a given substrate = more substrate is needed to achieve 1/2 Vmax malonate vs succinate on succinate dehydrogenase B. Noncompetitive inhibition the inhibitor and substrate bind at different sites on the enzyme => conformation change cannot be reversed by increasing [S] : decrease Vmax ?? Km is the same lead forms covalent bonds with the sulfhydryl side chains of cysteine ferrochelatase: protoporphyrin に Fe2+を附加してヘムを作る (Voet pp.438-440) δ-aminolevulinate: アミノレブリン酸 C. Enzyme inhibitors as drugs amoxicillin: C18H19N3O5S:経口投与される合成ペニシリン angiotensin: 血漿中に存在する 3 種のオリゴペプチド; 特にアンギオテンシン II、III は昇圧活性を持ち、副腎皮質を刺激し て、aldosterone を分泌させる angiotensin converting enzyme:血圧を上昇させる作用がある captpril: C9H15NO3S 抗高血圧薬として用いる白色の粉薬; ACE inhibitor の一つ enalapril:降圧薬;ACE 阻害 lisinopril:降圧薬;ACE 阻害 3/5 chpt 4. Enzymes VIII. Regulation of enzyme activity The rates of most enzymes are responsive to changes in substrate concentration because the intracellular level of many substrates is in the range of the Km. => first order A. Allosteric binding sites allosteric enzymes: regulated by effectors (a.k.a. modifiers or modulators) bind noncovalently at a site other than the active site alter the affinity of the enzyme or modify the maximal catalytic activity negative effector/positive effector Allosteric enzymes usually contain multiple subunits and frequently catalyze the committed step early in a pathway 1. Homotropic effector the substrate itself is an effector in most cases an allosteric substrate is a positive effector active sites bound to positive homotropic effectors, i.e. an allosteric substrate, are called cooperative cf. the binding of oxygen molecules to hemoglobin (Cell pp.173-175 fig.) sigmoidal plot 2. Heterotopic effector the effector is NOT a substrate ex. feedback inhibition B. Regulation of enzymes by covalent modification in most cases by the addition (phosphorylation) or removal (dephosphorylation) of phosphate groups from specific serine, threonine, or tyrosine residues of the enzyme (Cell p.176-) 1. Phosphorylation and dephosphorylation protein kinase utilizing ATP as a phosphate donor ≠phosphoprotein phosphatase 2. Response of enzyme to phosphorylation glycogen phosphorylase(degragede glycogen)...increases activity glycogen synthase (synthesize glycogen)...decreases activity C. Induction and repression of enzyme synthesis Cells can also regulate the amount of enzyme present, usually by altering the rate of enzyme synthesis rather than influencing the efficiency of existing enzyme molecules. => enzymes needed at only one stage of development or under selected physiologic conditions ex. insulin causes an increase in the synthesis of enzymes involved in glucose metabolism ≠ enzymes in constant use: not regulated by altering the rate of enzyme synthesis alternations in enzyme synthesis take hours to days, much slower than allosteric changes 4/5 chpt 4. Enzymes IX. Enzymes in clinical diagnosis plasma enzymes: 1) (a few) actively secreted by certain organs ex. zymogen of the enzymes involved in blood coagulation 酵素原,酵素前駆体:それ自体は酵素活性を持たないが,生体内でプロテアーゼによる切 断などの修飾を受けたとき酵素となる物質;proenzyme ともいう 2) (many) released from cells during normal cell turnover intracellular/no physiologic function in the plasma the presence of elevated enzyme activity in the plasma may indicate tissue damage accompanied by increased release of intracellular enzymes. A. Alternation of plasma enzyme levels in disease states The level of specific enzyme activity in the plasma frequently correlates with the extent of tissue damage. ex. heart, liver, skeletal muscle etc. useful in evaluating the prognosis for the patient B. Plasma enzymes as diagnostic tools alanine aminotransferase (ALT a.k.a. glutamate pyruvate transaminase; GPT) => abundant in liver GPT:肝細胞にある酵素で,その血中濃度は肝炎の指標 C. Isoenzymes and diseases of the heart isoenzymes(isozymes); catalyze the same reaction but differ in their physical properties ex. CPK(creatine kinase), LDH(lactate dehydrogenase), GOT(glutamate-oxaloacetate transaminase) => myocardial infarction when EKG is difficult to interpret creatine: C4H9N3O2:脊椎動物の筋肉にあるアミノ酸の一種 CK/CPK: 高エネルギー燐酸基をクレアチン燐酸から ADP に転移する反応を触媒する酵素 GOT: 血清や多くの身体組織, 特に 心・肝中に存在する酵素で, 組織傷害によって血清中に放出される 1. Quaternary structure of isoenzymes different subunits in various combinations ex. CK; CK1(BB), CK2(MB), CK3(MM)... dimer composed of two polypeptide 2. Diagnosis of myocardial infarction myocardial muscle: the only tissue containing more than 5% of the total CKs as CK2 CK2: appears 4 to 8 hours following onset of chest pain reaches a peak at 24 hrs LDH: peak 2 to 3 days after the onset of symptoms when CK2 decreases to the normal state 5/5