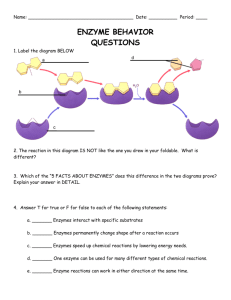
CP Biology Worksheet – Catalysts
advertisement

Catalysts 1. The energy needed to start a chemical reaction is called activation energy . 2. What is a catalyst? A catalyst is a compound that speeds up a chemical reactions 3. What are the main molecules that act as catalysts in living things called? enzymes 4. Enzymes are made of what kind of macromolecule? protein 5. Why are enzymes important in living things? Enzymes are very important to living cells because they allow chemical reactions necessary for life to occur in relatively moderate temperatures. Without enzymes, the chemical reactions needed to keep a cell alive would require such high temperatures to overcome the activation energy that the cell would be damaged or destroyed. 6. How do enzymes allow chemical reactions to happen faster? Enzymes speed up chemical reactions by lowering the activation energy required to get the reaction started. 7. How many different chemical reactions can a single enzyme catalyze? Enzymes are very specific, and generally only catalyze a single reaction. 8. The specific reactants acted upon by an enzyme are called substrates. 9. What is the active site of an enzyme? The active site is the region of the enzyme where the substrate (or substrates) fit together with the enzyme. 10. Explain how an enzyme interacts with its substrate. An enzyme may fit with its substrate in such way that it that it bends the substrate, weakening certain bonds, thus making it easier for them to break and create the products. Alternately, an enzyme may hold two substrates close together enabling them to react more easily. 11. Is an enzyme used up when it catalyzes a reaction? NO! A single enzyme molecule will be recycled over and over again.